Circumcision and
HIV: The AIDS Virus
Most HIV infection is via the foreskin
Over 25 million people have died from AIDS. UNAIDS figures show that in the year 2007 the death toll was 2.1 million [Sharlip, 2008]. So far approx. 60 million have been infected with HIV (13,000 each day, i.e., one every 7 seconds; 2.7 million in 2007). Half of all new infections are in people under 25 years of age. In all, approx. 33 million people (0.5% of the world population, or 1% of those aged 15-49) are currently living with HIV [www.avert.org/worldstats.htm] [Cohen, 2007b]. Half of these are women. Over 15 million children have been orphaned [www.unaids.org] [Pilcher, 2004; Ferrante et al., 2005]. By 2050 there could be one billion infected [Hale, 2001]! Of the half of all HIV cases that are men, most were infected through their penises [Kohn et al., 1999], the foreskin having been implicated as early as 1986 [Fink, 1986]. Over 80% of these infections were from vaginal intercourse [Joint, 1996].
The world is losing the fight against AIDS [Honey, 2007]. Globally, the number of people who will need anti-retroviral therapy after becoming HIV-positive will increase substantially [Mascolini et al., 2009]. For every one person put on therapy, 6 people get infected with HIV [Honey, 2007].
At the 4th International AIDS Society Conference in 2007 the Director of the National Institute of Allergy and Infectious Diseases and top advisor on HIV and AIDS to the President of the USA stated that there have been scientific advances that the research community should be proud of, namely the finding that male circumcision substantially reduces the risk of acquiring HIV [Honey, 2007]. The author of the present internet review chaired the Circumcision session at that conference.
On 28 March 2007 the World Health Organization and UNAIDS issued a statement endorsing circumcision in prevention of the spread of HIV [World, 2007]. This stated "the efficacy of male circumcision in reducing female to male HIV transmission has now been proven beyond reasonable doubt. This is an important landmark in the history of HIV prevention". It went on to recommend circumcision for men and boys. Infant circumcision was also advocated because it is "less complicated and risky".
The United Nations General Assembly has also noted the importance of male circumcision in the fight against AIDS [United, 2008].
The Centers for Disease Control and Prevention concluded in a Report in 2008 that "male circumcision may also have a role in the prevention of HIV transmission in the United States" [Centers, 2008a]. In 2009 it was reported in the New York Times on Aug 24 that the CDC wanted to promote infant circumcision in the USA in order to protect against HIV and other conditions caused by lack of circumcision [Rabin, 2009]. In Jan 2010, the outcome of a 2 day consultation held in April 2007 with a broad spectrum of stakeholders, was published in Public Health Reports [Smith et al., 2010]. This stated "Consultants suggested that (1) sufficient evidence exists to propose that heterosexually active males be informed about the significant but partial efficacy of MC in reducing risk for HIV acquisition and be provided with affordable access to voluntary, high-quality surgical and risk-reduction counseling services; (2) information about the potential health benefits and risks of MC should be presented to parents considering infant circumcision, and financial barriers to accessing MC should be removed".
"Circumcision Can Prevent HIV" was chosen as #1 in the "Top Medical Breakthroughs for 2007" by Time magazine [http://www.time.com/].
How then does HIV enter a man's body during heterosexual intercourse? As will be discussed later in this section, epidemiological data from more than 45 studies shows that HIV is much more common in uncircumcised, as opposed to circumcised, heterosexual men [Fischbacher, 1999]. There is in fact now a wealth of evidence to indicate that male circumcision protects against HIV infection, as acknowledged in editorial commentaries and reviews in the major journals Science [Jha et al., 2001; Cohen, 2005b; Cohen, 2005a; Cohen, 2008c; Potts et al., 2008], Nature [Weiss, 2001; Anonymous, 2007a; Check, 2007a; Fauci, 2007a], the New England Journal of Medicine [Katz & Wright, 2008], the Lancet [Anon, 2007; Newell & Barnighausen, 2007; Cates & Feldblum, 2008], as well as a host of other good medical journals [Chan, 2006; Fauci, 2007b; Flynn et al., 2007; Hargreaves, 2007; Jones, 2007; Madon et al., 2007; Molokwu, 2007; Moszynski, 2007; Peltzer et al., 2007; Quinn, 2007; Roehr, 2007; Wakabi, 2007; Weiss, 2007; Anonymous, 2008a; Aral et al., 2008; Cohen et al., 2008; Gray et al., 2008; Hargrove, 2008; Laurencin et al., 2008; McKinney et al., 2008; Sharlip, 2008; Weiss et al., 2008a; Weiss, 2008; Willyard, 2008; Auvert et al., 2009c; Doyle et al., 2009; Gray, 2009b; Mascolini et al., 2009; Moses, 2009; Vergidis et al., 2009; Vermund et al., 2009; de Bruyn et al., 2010], a Harvard report [Burrell, 2008], the US Congress [Waxman, 2008] and a report compiled by the World Bank [Wilson & de Beyer, 2008]. Male circumcision is the most powerful intervention available currently in the fight against AIDS [Sharlip, 2008; Klausner et al., 2008]. The promotion of circumcision for HIV prevention has consequently been widely advocated [Drain et al., 2004].
Circumcision represents a surgical 'vaccine' in the face of the dismal failure of two decades of research to develop a conventional vaccine [Anonymous, 2007a; Anonymous, 2007b; Cohen, 2007d; Cohen & Lester, 2007; HIV, 2007; Anonymous, 2008b; Barouch, 2008; Check Hayden, 2008; Cohen, 2008b; Cohen, 2008c; Potts et al., 2008; Walker & Burton, 2008; Weiss, 2008; Cohen, 2009c; Cohen, 2009b; Gray, 2009b; Mascolini et al., 2009]. In fact on Nov 8, 2007 one vaccine was widely reported to actually INCREASE risk of HIV infection [Cohen, 2007a; Fox, 2007; Anonymous, 2008b; Check Hayden, 2008; Weiss, 2008; Gray, 2009b]. And infection was noted as being greater in uncircumcised men. For example in a vaccine trial published in the Lancet, the circumcised men in the trial exhibited 74% lower HIV acquisition [[Buchbinder et al., 2008]. In 2009 the "as-treated" data from a large and expensive trial in Thailand indicated only a small, nonsignificant reduction in HIV infections [Cohen, 2009a]. Currently, a conventional vaccine for the prevention of HIV appears to be near impossible [Addanki et al., 2008].
To date, most of the emphasis has been placed on three “established” pillars of HIV prevention, namely condom promotion and distribution, voluntary counselling and testing, and treatment for other sexually transmitted infections (STIs) [Shelton, 2007], with the teaching of abstinence being added by the US global AIDS program. Have these worked?
Microbicides, once touted as an answer, have also been a failure [Cates & Feldblum, 2008; Gray, 2009b; Mascolini et al., 2009]. For example, Phase III trials of Savvy gel and C-31G failed, and the spermicide nonoxynol-9 [Check, 2007b], as well as a cellulose sulfate gel, Ushercell, actually increased risk of becoming infected with HIV [Anon, 2007; Anonymous, 2007d; Anonymous, 2007a; Check, 2007b; Ramjee et al., 2007]. The latter trial, involving 1330 women in 4 countries, was stopped in Feb 2007. In 2008 Carraguard, containing carrageenan, was found to be safe, but not effective against HIV, in a $40 million placebo-controlled trial in South Africa [Cohen, 2008e]. The women in the trial used it only 44% of the time, with a mere 10% using it always prior to sex. In late 2009 a large placebo-controlled trial of PRO 2000 in 4 African countries showed 130 infections in those who got the active gel and 123 in those who were given the placebo.
Voluntary counselling, teaching abstinence, and treatment of other STIs have had little impact on HIV infection rates [Potts et al., 2008]. Condom use has not reached a sufficiently high level, even after many years of widespread and often aggressive promotion, to slow the spread of HIV in general populations [Potts et al., 2008], as will be discussed in more detail later in this section.
The best that can be done for HIV-infected people is chronic suppression of HIV replication by highly active antiretroviral therapy (HAART), but this strategy is limited by high cost, the requirement for lifelong adherence and the unknown effects of long-term treatment [Richman et al., 2009]. Urological complications seen in patients receiving HAART therapy include urinary tract infections, urolithiasis (kidney stones leading to acute renal failure) seen in 5-25% of patients given indinavir, and benign prostatic hyperplasia and prostate cancer [Heyns et al., 2009]. Toxicity and drug resistance, especially in poorer countries where antiretroviral regimens are substandard as well as the fact that at the end of 2007 there were 6.7 million people in need of the drugs are of conern [Cohen, 2008a]. By 2015 it will cost US$41 billion annually to treat the 13.7 million people with CD4+ counts of less than 200 [Cohen, 2008a]. Since wealthy countries donate US$10 billion currently, a substantial shortfall is evident. According to Geoffrey Garnett, an epidemiologist at the Imperial College in London, antiretroviral drugs are unlikely to make a large impact on HIV globally, since roughly 80% of people who get infected do not know their HIV status, and most who do are not eligible for free treatment until their immune systems have been substantially damaged, meaning that transmissions occur long before people are taking the drugs [Cohen, 2008d]. Garnett suggested instead creating natural synergy by combining treatment with condoms and circumcision. Prevention of infection is obviously preferred to expensive treatment having side effects and limited effectiveness.
Biological basis of the protective effect of circumcision
against HIV infection
The history of HIV research has been summarized by Professor Robin Weiss, University College, London [Weiss, 2008].
During heterosexual intercourse the foreskin is pulled back down the shaft of the penis, meaning that the whole of its inner surface is exposed to vaginal secretions [Szabo & Short, 2000]. An early suggestion that attempted to explain the higher HIV infection in uncircumcised men was that the foreskin could physically trap HIV-infected vaginal secretions and provide a more hospitable environment for the infectious inoculum [Cameron et al., 1989]. It was also suggested that the increased surface area, traumatic physical disruption during intercourse and inflammation of the glans penis (balanitis) could aid in recruitment of target cells for HIV-1. Initial thoughts were that the port of entry could potentially be the glans, sub-prepuce and/or urethra. It was suggested that in a circumcised penis the drier, more keratinized skin covering the penis may prevent entry. However, subsequent studies showed that the glans of the circumcised and uncircumcised penis were in fact identical in histological appearance, having exactly the same amount of protective keratin [Szabo & Short, 2000]. There is evidence that, in contrast, the inner lining of the foreskin is a mucosal epithelium and lacks a protective keratin layer [Bailey, 2001] (see picture below taken, with permission, from Bailey [2001]).
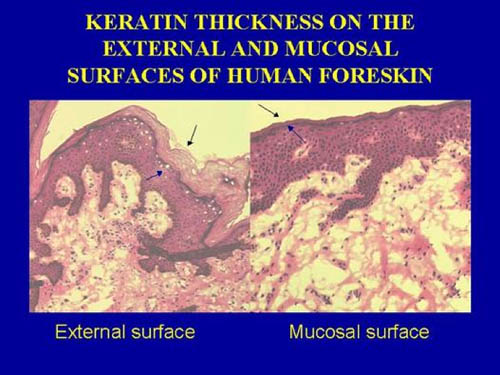
The foreskin's inner epithelium would thus resemble histologically the lining of the nasal passages and vagina. All such mucosal epithelia are major targets for infection by micro-organisms (colds, flu, STIs, etc). A study that found no difference in keratinization of the inner and outer layers of foreskins from 16 adult male donors undergoing elective circumcision in Chicago [[Dinh et al., 2010] has been criticized because (1) the foreskins were from men circumcised for foreskin pathologies that could have increased keratinization and (2) the samples could have been from the distal end of the foreskin that is thicker than the proximal foreskin near the coronal sulcus [R.H. Gray and R.C. Bailey, personal communication]. The uncircumcised penis is more susceptible to minor trauma and ulcerative disease, and the preputial sac could harbor pathogenic organisms in a pool of smegma [Alanis & Lucidi, 2004]. The mucosal inner lining of the adult foreskin is rich in Langerhans cells and other immune-system cells (22.4, 11.5 and 2.4% of total cell population is represented by CD4+ T cells, Langerhans cells and macrophages, respectively) [Patterson et al., 2002].
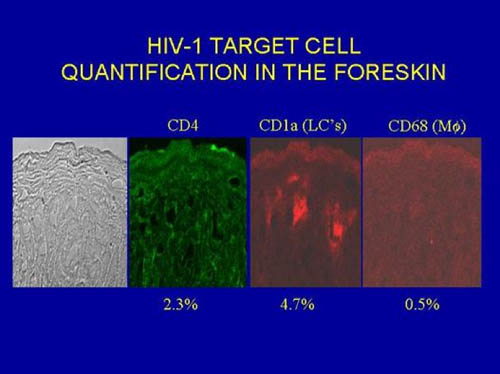
(This contrasts with the neonate, where the foreskin is deficient in such cells [Weiss et al., 1993], the proportion being instead 4.9, 6.2 and 0.3%, respectively [Patterson et al., 2002]. The respective percentages for immune-system cells in the cervical mucosa are: 6.2, 1.5 and 1.4% [Patterson et al., 2002]. In the external layer of the foreskin, which is like the rest of the penis, the proportions are very much lower: 2.1, 1.3 and 0.7%, respectively [Patterson et al., 2002]. Although the urethra is also a mucosal surface, Langerhans cells are rarer, and it is not regarded as a common site of HIV infection. Chinese preschool boys have higher Langerhans cell density and lower keratinization in the thin foreskin as compared to adults [Qin et al., 2009].
For uncircumcised men, those with a higher foreskin surface area are more likely to be infected with HIV [Kigozi et al., 2009b], so adding to the evidence that the foreskin is an important factor in acquisition of HIV. Area was 43 square centimeters in those who acquired HIV and 37 square centimeters in those who did not (P = 0.01). HIV incidence was 0.80 per 100 person years for men whose foreskin area was in the lowest quartile (< 26 square centimetres), and was 2.5 per 100 person years in men whose foreskin area was in the highest quartile (>46 square centimetres), a 4-fold difference.
The counterintuitive observation that HIV risk is actually lower in circumcised men who have more frequent exposure than it is in circumcised men with less frequent exposure, has led to the hypothesis that repeated contact of the small area of exposed urethral mucosa, or more likely the meatus, which unlike the urethra does contain a small number of HIV receptors [McCoombe & Short, 2006], with subinfectious inoculums may induce an immune response having a protective effect over and above that afforded by removal of the vulnerable foreskin [Wawer et al., 2005]. The small area exposed may mean that the infectious inoculum per act of intercourse may be less likely to overwhelm the effects of partial protection as compared with the mucosal area exposed in a foreskin or vagina [Wawer et al., 2005]. This hypothesis remains to be tested. Mucosal alloimmunization has also been suggested as a protective factor against HIV [Peters et al., 2004].
Human genital (ectocervical) epithelial cells (in cell culture in the lab) can capture HIV on their surfaces and maintain it in a fully infectious state for at least 6 days [Wu et al., 2003]. It is also of interest, as far as transmission from the male to a sexual partner is concerned, that although HIV's infectivity in vitro is low, the 248-286 peptide fragment of prostatic acid phosphatase (a major constituent of semen) is present in semen at approx. 35 ug/ml and, by forming fibrils known as se men-derived enhancer of viral infection (SEVI), increases infectivity 100,000 fold by enhancing the attachment of HIV to target cells [Münch et al., 2007; Roan & Greene, 2007].
The immune cells of the inner lining of the foreskin help fight bacteria and viruses that accumulate under it. However, in the case of HIV, they act as a “Trojan horse”, serving as portals for uptake of HIV into the body, where HIV entry generally requires CD4 receptors and cofactors such as chemokine receptors CCR5 and CXCR4 present in high density on the surface of Langerhans cells [Alanis & Lucidi, 2004]. Although cells with HIV receptors CD1a, CD4, CCR5, CXCR4, HLA-DR and DC-SIGN are present throughout the epithelia of the inner lining, HIV can only infect those cells it can gain access to. Most Langerhans cells are in the epithelium closest to the surface of the inner foreskin lining (1.2% vs 0.3% of cells), whereas macrophages are mainly in the submucosa (0.04% vs 0.02%) [Donoval et al., 2006]. CD4+ T cells are present in each region. The inner layer is thus highly susceptible to HIV infection. What’s more, Langerhans cells send up dendritic projections (which resemble tentacles or “little fingers”) up between the keratinocytes (the other cells in the epithelia). These are particularly superficial in the inner foreskin (4.8 micrometers) compared with the outer foreskin (20 micrometers) [McCoombe & Short, 2006] (see diagram). In fact they are even closer than this owing to some retraction during transport to the lab for analysis (Scott McCoombe, personal communication).
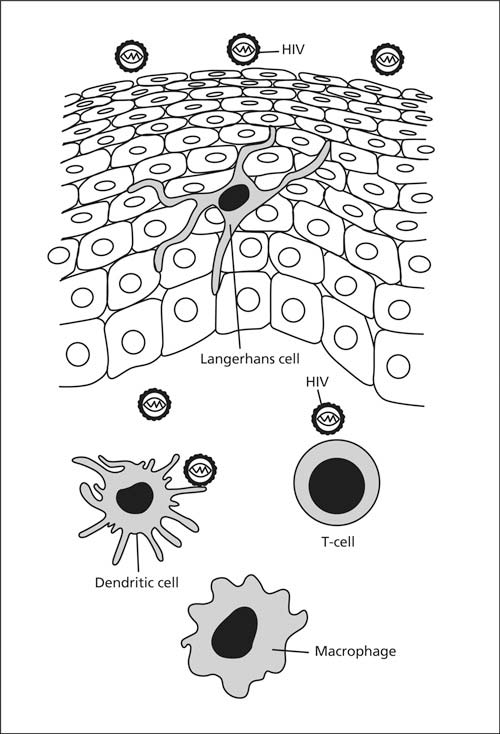
The selective entry of HIV via the thin, moist inner foreskin has been shown by direct experimentation [BBC2., 2000; Bailey, 2001; Patterson et al., 2002]. Punch biopsies were taken from fresh foreskin obtained immediately after circumcision of the adult male. Cultures were made of cells from the external surface (which resembles the rest of the penis) and from the inner mucosal surface of the foreskin. Live HIV tagged with a fluorescent marker was then applied. Within minutes the HIV entered the Langerhans cells (see diagram below - obtained, with permission, from Bailey [2001]; similar images can be seen in Patterson et al. [2002]). No uptake occurred for cultured epithelium of the outer surface of the foreskin, i.e., the part that resembles the skin of the circumcised penis. The mean number of HIV copies per 1,000 cells (determined by quantitative PCR) one day after infection was 301 for the inner foreskin, but was undetectable in the outer, external, foreskin [Patterson et al., 2002]. For cervical biopsies mean HIV copy number was 30, showing that the inner foreskin is 10-times more susceptible to HIV infection than the cervix [Patterson et al., 2002]. The HIV receptor CCR5 was, moreover, especially prevalent on foreskin tissue cells [Patterson et al., 2002]. This biological work thus nicely confirms the epidemiological evidence to be discussed below. It is furthermore supported by experiments in which SIV (the monkey equivalent of HIV) was applied to foreskin of monkeys, that then became infected [Miller, 1998]. The monkey work also showed infected Langerhans cells. Antigen presenting cells in the mucosa of the inner foreskin [Hussain & Lehner, 1995] are a primary target for HIV infection in men [Szabo & Short, 2000]. Differing results for explant culture have, however, been obtained by others, who noted nonetheless that “circumcision would remove two out of three of the exposed surface areas of the penis, reducing the chance of the virus coming into contact with susceptible target cells” [Fischetti et al., 2009].

Several mechanisms are involved in internalization of HIV [Turville et al., 2002; Boggiano & Littman, 2007]. One involves the c-type lectin, Langerin, present in Langerhan’s cells, which can bind HIV, internalize it and is then involved in its transport to regional lymph nodes [Turville et al., 2002]. Other mechanisms are more important [Boggiano & Littman, 2007]. There is also direct infection of T cells by HIV, independent of Langerhans cells [Boggiano & Littman, 2007]. It could be, however, that HIV’s success in establishing a systemic infection might depend on its early interaction with Langerhan’s cells [Boggiano & Littman, 2007]. At low viral levels Langerin is able to clear HIV, shunting it to intracellular granules for degradation, but this mechanism becomes overwhelmed at higher viral loads [de Witte et al., 2007; Schwartz, 2007].
Importantly, however, there is no need for passage of HIV through Langerhans cells for infection of T cells to occur [Hladik et al., 2007]. In the vaginal mucosa HIV enters CD4+ T cells almost exclusively by CD4 and CCR5 receptor-mediated direct fusion, leading to overt productive infection [Hladik et al., 2007]. In contrast, entry of HIV into CD1a+ Langerhans cells occurs primarily by endocytosis, involving multiple receptors, and the virions persist intact within the cytoplasm for several days [Hladik et al., 2007].
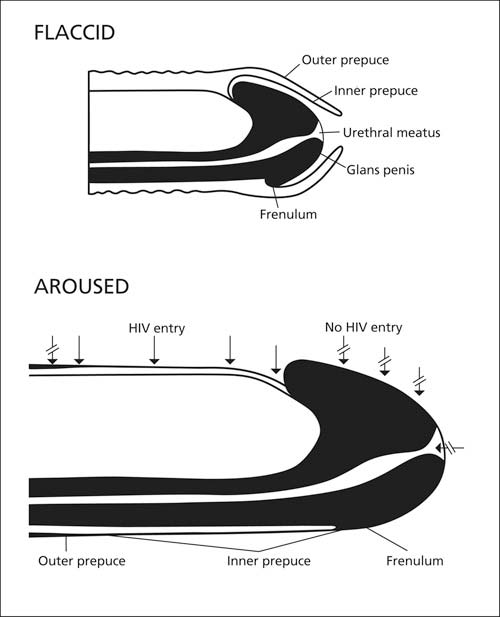
When the penis is aroused the inner lining of the foreskin becomes stretched halfway down the shaft (see diagram below modified from McCoombe & Short [2006]). Its thin keratin lining becomes even thinner as a result, and when inserted into the vag1na or rectum of an infected partner the vulnerable inner foreskin becomes wholly and directly exposed to infected fluids in the partner. After intercourse, having acquired HIV, the preputial cavity serves as a hospitable environment for the infectious inoculum. This then facilitates transmission to subsequent sex partners.
A report in 2010 identified the entire microbiome of the penis of 12 men before and after circumcision [Price et al., 2010]. Among the 42 unique bacterial families identified, Pseudomonadaceae and Oxalobactericeae were the most abundant irrespective of circumcision status. Circumcision was associated with a significant change in the overall microbiota (P = 0.007) and with a significant decrease in putative anaerobic bacterial families (P = 0.014). Two families in particular - Clostridiales Family XI (P = 0.006) and Prevotellaceae (P = 0.006) - were uniquely abundant before circumcision. Within these families the authors identified a number of anaerobic genera previously associated with bacterial vaginosis including: Anaerococcus spp., Finegoldia spp., Peptoniphilus spp., and Prevotella spp. The researchers concluded that "the anoxic microenvironment under the foreskin may support pro-inflammatory anaerobes that can activate Langerhans cells to present HIV to CD4 cells in draining lymph nodes". They suggested that the reduction in putative anaerobic bacteria after circumcision may play a role in protection from HIV and other sexually transmitted infections.
The foreskin is thus the weak point that allows HIV to infect men during heterosexual intercourse with an infected partner. A circumcised man with a HEALTHY penis is thus very unlikely to get infected. However, ulcerations (from herpes, syphilis, etc) or abrasions on the penis will allow infection and a circumcised man with these will continue to be at risk of HIV, as well as some other STIs. In an early study, individuals with HSV-2 have twice the risk of acquiring HIV than those without, and those infected with both viruses are more likely to transmit HIV than if they just have HIV [Stephenson, 2004]. In another the risk was increased 3-5 fold [Todd et al., 2006]. Giving co-infected patients acyclovir was therefore suggested. Interventions that treat curable STIs are cost-effective in HIV prevention in populations that engage in high-risk behaviors or have low circumcision rates [White et al., 2008b]. But further research to be discussed below argues against this. Of course condom use is strongly advocated in attempting to lower transmission. Condoms, when ALWAYS used, reduce HIV infection by 80-90% [Halperin et al., 2004]. Condom use remains low, however [Ferrante et al., 2005]. In one study, 78% had never used them [Yahya-Malima et al., 2007]. Another, in Australia, found 25% never used them, with only 25% always using them [Kang et al., 2006]. (Findings from other research on condom use can be found in a later section.) Moreover, condoms are not a panacea, and a man with a foreskin can still be infected by HIV-laden fluids coming into contact with the inner foreskin, for example during foreplay, prior to application of the condom preceding vaginal penetration. A condom can, moreover, break!
Epidemiological Research
HIV appeared first in Sub-Saharan Africa, quite likely in the Belgian Congo, in the 1930s or 40s [Holmes, 2007]. From there HIV subtype B moved to Haiti between 1961 and 1970 [Gilbert et al., 2007]. This is a period when many Haitians returned to their home country after the Congo’s independence from Belgium. It spread within Haiti for some years before dispersing elsewhere in the world between 1966 and 1972. The Americas were probably the first after Haiti to be recipients. HIV circulated cryptically in the USA for about 12 years before AIDS was recognized as a new disease in 1981. The emergence of a pandemic variant of subtype B was an important turing point in the history of AIDS, but its spread was likely driven by ecological rather than evolutionary factors.
A joint WHO/UNAIDS report back in 1998 found the percent of 15-49 year-olds infected with HIV in different regions was as follows: Sub-Saharan Africa 8.6%, Caribbean and Latin America 0.6%, South and SE Asia 0.6%, ‘Western’ countries 0.4%, Eastern Europe and Central Asia 0.2%, North Africa and Middle East 0.1%, East Asia and Pacific 0.1% [Gerbase et al., 1998].
In a 2006 report, UNAIDS figures showed that HIV prevalence in adults in 29 developing countries (not including Sub-Saharan Africa) with primarily heterosexual transmission was 0.76% for 11 with low (<20%) rates of circumcision and 0.09% for 17 with high rates of circumcision (>80%) [Drain et al., 2006], i.e., was 8-fold higher in those with low circumcision rate. The difference was highly significant (P <0.001). Similar analyses by others have confirmed the inverse correlation between HIV prevalence in different countries and their rate of circumcision [Addanki et al., 2008].
Heterosexual transmission is the primary mode of infection in Africa, the Middle East, the Caribbean, India and in parts of Asia. Lower rates were seen in predominantly Christian countries that practice circumcision such as the Philippines, Benin, Ghana, Equatorial Guinea and Gabon. Data for Sub-Saharan Africa appears in a later section. What follows summarizes data for various countries, both developing and developed.
In developing countries the rate of female-to-male HIV transmission was found to be 341 times higher than in developed countries [O'Farrell, 2001]. (This compared with a male-to-female rate 2.9-fold higher in developing countries.) Among couples in the West, female-to-male transmission was 11% [Mastro & de Vincenzi, 1996]. For male-to-female it was 23%. In Africa, however, female-to-male was 73% [Hira et al., 1990] and male-to-female was 60% [Latif et al., 1989; Hira et al., 1990]. In another study, in rural Uganda, female-to-male transmission (12 per 100 person years) was identical to male-to-female transmission [Quinn et al., 2000]. A systematic review and meta-analysis in 2009 of 43 publications comprising 25 study populations found female-to-male transmission in high-income countries (North America, Europe) was 0.04% per sex act and male-to-female was 0.08% per sex act [Boily et al., 2009]. In low-income countries (Africa, Asia, Haiti) female-to-male was 0.38% and male-to-female was 0.30%. These data were for the absence of commercial sex exposure. In comparison, for receptive anal intercourse it was 1.7% per act.
After consideration of all of the factors, lack of circumcision was highlighted as a major driving force behind the AIDS epidemic [O'Farrell, 2001].
United States of America:
According to the Centers for Disease Control and Prevention and other sources there have been 1.7 million HIV infections and 1.1 million AIDS cases in the USA to the end of 2006, with 0.54 million deaths [Centers, 2008b; Holtgrave et al., 2009]. HIV prevalence in those aged 18-39 was 0.38% in 1988-1994 and was 0.37% in 1999-2002 [McQuillan et al., 2006]. Prevalence is 12.8 per 100,000 population. In 2006 there were 56,300 new HIV infections [Centers, 2008b; Hall et al., 2008b]. Of these, 77% were in men. High-risk heterosexual contact accounted for 31-33% of HIV/AIDS cases diagnosed in 2006 [Centers, 2008b; Xu et al., 2009]. Heterosexual exposure is becoming the leading mode of HIV transmission, representing, for example, 38% of incident cases among youths aged 13-24 years in Washington, DC [Government of the District of Columbia, 2008]. Men comprised 36% and women 64% of heterosexually-acquired infections in 2007 [Centers, 2008b]. Heterosexual contact was, moreover, reported to be responsible for 15% of HIV infections in men in 2005 [US, 2007]. In New York City, the epicenter of the HIV epidemic, HIV prevalence was 2.1% among male STI clinic patients [McKinney et al., 2008]. Homosexual sex accounted for 59% of infections in the USA [Centers, 2008b]. Of women, 65% were infected during heterosexual contact. There were 28 children aged under 13 years who had HIV, most having acquired the virus from their mothers. New AIDS cases in children under 13 in 2007 numbered 28 and there were 9,209 cumulative AIDS cases to the end of that year [Centers, 2008b].
The number of diagnoses increased annually to 2005, reaching 45,669 in that year, with 17,011 deaths. In total 550,394 have died since the beginning of the epidemic, most prior to age 45. Heterosexual acquisition has grown by 42%. The incidence figures may, however, be underestimates, the true numbers likely being twice these.
In the USA an early overall estimate of risk of HIV infection PER heterosexual exposure, when HIV status is unknown, was less than 1 in 100,000 [Caldwell & Caldwell, 1996; Padian et al., 1997]. A report in 2008 concluded that, OVER THE LIFETIME, risk to a male in the USA is 1 in 53 and to a woman 1 in 140 [Hall et al., 2008a]. It was higher in people in their 30s, and differed by race, being 1 in 16 for Black males, 1 in 35 for Hispanic males, and 1 in 104 for white males.
An association of higher incidence of HIV with being uncircumcised in the USA was first noted in 1989 [Whittington, 1989]. A study of heterosexual couples in Miami found a higher incidence of HIV in men who were uncircumcised. A study in New York City found that risk ratio for HIV infection in heterosexual men as a result of being uncircumcised was 4.1, rate being 2.1% versus 0.6% for uncircumcised men as compared circumcised men [Telzak et al., 1993]. Another US study that looked at heterosexual sex found a risk ratio of 2.9 [Kassler & Aral, 1995]. (See also review by Moses et al. [1998].)
In heterosexual African American men attending STI clinics in Baltimore from 1993 to 2000, the prevalence of HIV was 2.7%. Looking at the data for those with known exposure to HIV, infection was seen in 10.2% of the men who were circumcised, compared with 22.0% of the men who were uncircumcised (adjusted prevalence ratio = 0.49) [Warner et al., 2009].
In homosexual men a study in Seattle found a 2.2-times higher HIV-positivity in the 15% who were uncircumcised [Kreiss & Hopkins, 1993]. A later study in Seattle found no difference, however [Jameson et al., 2009]. But caution is needed in interpretation of the latter results, since they were derived from men attending a STI clinic. Since circumcised men are protected from STI because of being circumcised they will be less likely to attend an STI clinic, meaning data from STI clinic cohorts are biased, i.e, such data do not necessarily represent the prevalence of an STI amongst men of each circumcision status in the general population. The bias is, moreover, away from showing a protective effect of circumcision. Another study, involving 3,257 homosexual men in 6 US cities studied from 1995-1997, identified various risk factors, lack of circumcision once again being found to double the risk of acquiring HIV [Buchbinder et al., 2005]. A failed HIV vaccine trial stopped in 2007 noted that "infected men were less likely to be circumcised" [Fox, 2007]. No association between circumcision status and either HIV or syphilis infection in homosexual men was seen in a San Francisco study, although the authors noted that a large proportion of gay men practice both insertive and receptive anal intercourse [Mor et al., 2007]. The latter would dampen the possibility of seeing an association with circumcision status.
Interestingly, per-contact risk of infection from receptive oral sex is claimed to be comparable to that of insertive anal sex [Vittinghoff et al., 1999; Celum et al., 2001; Buchbinder et al., 2005]. This is not, however, generally accepted as yet.
The relatively moderate HIV infection rate in the USA is likely contributed by the high prevalence of circumcision in this country [Addanki et al., 2008].
A position paper in 2007 stated “it is likely that circumcision will decrease the probability of a man acquiring HIV via penile-vaginal sex with an HIV-infected woman in the USA” and that “some sexually active men may consider circumcision as an additional HIV prevention measure” [Sullivan et al., 2007]. This was in addition to condoms of course, although in the USA condoms were never used during heterosexual sex with a non-primary partner in the case of 16% of men and 24% of women [Sanchez et al., 2006].
The Report warned, however, that any reduction in reimbursement by public and private medical insurance for circumcision, and any decline in rate of circumcision, could reverse the benefit that the USA has enjoyed to date because of its high rate of circumcision [Sullivan et al., 2007].
As mentioned above, the Centers for Disease Control announced in 2009 that it would be recommending infant male circumcision for prevention of HIV and other medical conditions (New York Times, Aug 24, 2009) [Rabin, 2009], thus answering those who had been equivocating on this issue [Xu et al., 2009].
Canada:
Approx. 33% of new HIV infections diagnosed in Canada in 2006 were from heterosexual contact [UNAIDS, 2008].
United Kingdom:
In the United Kingdom and several other Western European countries, most infections are from heterosexual contact. The incidence in the UK is 1.4 per 100,000 or 0.2% of the population [www.avert.org/aids-uk]. There has been a call for the the UK to revise its male circumcision guidelines and for the National Health Service (NHS) to make circumcision available, especially for recent male immigrants from high HIV-prevalence countries and men who have sex with men [Macdonald et al., 2008].
Australia:
In Australia there were 27,331 diagnoses of HIV and 10,303 diagnoses of AIDS up until the end of 2007, with 6,767 deaths [http://www.avert.org/ausstatg.htm]. To the end of 2008 diagnoses were 28,330. AIDS incidence is 0.9 per 100,000. In the past decade the number of HIV infections have risen steadily from 645 in 1998 to 995 in 2008, the increase over the past 10 years being 38%. In women a steady rise occurred from 73 in 1988 to 140 in 2007. Sex between men is the highest category, but fell to 64% of the total by 2007. In contrast the proportion of infections from heterosexual sex rose to 21% in 2007. Injecting drug use accounted for 3% of infections and the remaining 4% was in homosexual drug-injecting men. The rate of HIV was highest in New South Wales (6.2 new diagnoses per 100,000 people in 2007), followed by Victoria (5.5, a doubling in the past 10 years), Queensland (4.6), SA (3.6) and WA (3.6). Although most men got circumcised as babies until the 1970s, the downward trend thereafter would be expected to contribute to a rise in HIV/AIDS in coming decades as more and more of these younger males enter the pool of sexually active adults and adolescents [Addanki et al., 2008]. The high level of unprotected sex and failure of schools to address the risk posed by STIs (including basing messages, particularly in Catholic schools, solely on abstinence) can only mean ongoing rises in infections.
Western Europe:
UNAIDS estimated that approx. 2.2 million people were living with HIV at the end of 2007 [http://www.avert.org/hiv-aids-europe.htm]. The proportion in Western Europe was half that in Eastern Europe. In one report, unprotected heterosexual intercourse accounted for 42% of new HIV diagnoses in Western Europe in 2006, compared to 29% attributable to men who have sex with men [UNAIDS, 2008]. In another, of the 24,202 people diagnosed with HIV in Western Europe in 2007, 51% had probably acquired their infection from heterosexual contact, 40% were in men who have sex with men, and 8% were in injecting drug users [http://www.avert.org/hiv-aids-europe.htm]. For the 16 Western countries that have consistently reported data since 2000, the highest rates in 2007 were Portugal (21.7/100,000), the UK (10.8/100,00) and Switzerland (10.2/100,000). Circumcision has been advocated in Europe as part of a package for HIV prevention [Weidner, 2007].
Central Europe:
There were 1,897 HIV diagnoses in Central Europe in 2007, 53% being from heterosexual contact, 30% in men who have sex with men, and 13% amongst injecting drug users [http://www.avert.org/hiv-aids-europe.htm].
Russia, Eastern Europe, and Central Asia:
The prevalence of HIV has doubled in Russia, Eastern Europe and Central Asia between 2001 and 2007, making this region the world's most rapidly expanding epidemic [http://www.avert.org/aids-russia.htm] [Pilcher, 2004]. In 2007 there were approx. 1.5 million people in Russia, Eastern Europe and Central Asia living with HIV, and the number who became infected that year was 110,000; as well there were 58,000 deaths from AIDS in 2007 [http://www.avert.org/aids-russia.htm]. Russia + Ukraine account for 90% of infections, with 1.6% of Ukrainians and 1.1% of Russians infected. High prevalence is also seen in Estonia (1.3%) and Latvia (0.8%). In Russia, heterosexual transmission grew from 5% of all infections in 2001 to 20% in 2005 [http://www.avert.org/ecstatee]. Two-thirds of infections in women are from heterosexual contact [http://www.avert.org/aids-russia.htm]. Amongst truck drivers in Azerbaijan, HIV was 2.7 times higher in uncircumcised drivers, with 2.9% of Russian drivers, 1.7% of Ukrainian drivers and 1.1% of Azerbaijan drivers being infected [Botros et al., 2009]. Even back in 1993, for Europe generally (13 centers from 9 countries) rate was higher than in the USA: 3 in 10,000 [Downs & de Vincenzi, 1996]. (And circumcision rate is very low in Europe.) The proportion of infections due to female to male transmission in Europe is much higher than in the USA, consistent with the influence of the much lower rate of circumcision in Europe [Halperin & Bailey, 1999; Gray et al., 2000b; Wamai et al., 2008].
Asia in general:
Asia has 5 million people living with HIV [http://www.avert.org/aids-asia.htm] In Asia, rate of HIV is low where circumcision is high: e.g., Philippines (0.06% of adults are HIV-positive), Bangladesh (0.03%) and Indonesia (0.05%). In contrast the rate is 10-50 times higher in countries with a large proportion of uncircumcised males: e.g., Thailand (2.2%), India (1.8%) and Cambodia (2.4%) [Halperin & Bailey, 1999]. The high circumcision rate in the Philippines has kept HIV rates low [Normile, 2009]. Large increases in infections are expected in such Asian countries over time [Halperin & Bailey, 1999]. In recent years there has been a surge in infections amongst homosexual men [Normile, 2009]. More details for each region follow:
China:
To the end of 2007 China had 0.7 million people living with HIV (0.1% of the adult population of this country whose total population is 1.3 billion) [http://www.avert.org/aids-asia.htm]. This represented an 8% increase since the previous year and the epidemic continues to expand [Lu et al., 2008]. The prediction of 10 million HIV cases by 2010 [www.avert.org/aidschina] was realistic. The Yunan province in southwest China is where HIV was first detected, in intravenous drug users, in 1989 [Zheng et al., 1994]. A contributing factor to an outbreak of HIV in central China in 2000 was use of contaminated needles to buy and on-sell blood from people there. Whereas in 2005 11% of infections were from heterosexual contact, by 2006 this proportion had jumped to 38% [Lu et al., 2008]. The proportion in drug users has dropped from 100% in 1989 to 40% in 2006 [Lu et al., 2008]. The leadership of this, the biggest country in the world, is well placed by its political ideology to reduce such a disaster by institution of a circumcision policy. Since circumcision rate in China is low, the promotion of circumcision in China has been suggested [Ben et al., 2008; Ben et al., 2009]. In recognition of the need for China to implement circumcision to reduce sexually transmitted infections and disease a call has been made to establish surgical standards and training protocols for the promotion of circumcision in China [Li et al., 2009].
India:
HIV was first reported in India in 1986 and is now widespread. An early report said 5.1 million were infected (1% of the adult population [Godbole & Mehendale, 2005] or 0.5% of the total population [www.avert.org/aidsindia]). However, this was revised downwards in 2007 to 2.5 million (0.4% of adults) [Cohen, 2007c]. Nevertheless the figure could be up to 6 million [Cohen, 2007c]. Because of its large population India may have one of the highest number of persons with HIV in the world. HIV prevalence is expected to reach 1.9% of the population by 2019 [Addanki et al., 2008]. The population of India is over one billion and only 15% of men (mostly Muslims) are circumcised. Most of the population is Hindu, and Hindu men are virtually all uncircumcised, putting them at increased risk. A prospective study published in the Lancet in 2004 of 2,298 men initially not infected with HIV found that circumcision was strongly protective against HIV-1 infection, with a 6.7-fold reduction in adjusted relative risk (0.14; P = 0.0089) [Reynolds et al., 2004]. The data led the authors to conclude that biological rather than behavioral differences were responsible and that the foreskin has an important role in sexual transmission of HIV. Most infections in India are from heterosexual sex. A study published in 2007 found lower HIV incidence in Muslim men (circumcised) compared with Hindu men (uncircumcised), 1.0% versus 4.4%, respectively (odds ratio 0.42), despite Muslim men having more sex partners and visits to commercial sex workers [Talukdar et al., 2007]. This finding was not influenced by concurrent infection with other STIs. A study in Mysore in 2008 of an 89% Hindu population found HIV in 1.1% of 1,732 uncircumcised men but 0% of 193 circumcised men [Munro et al., 2008]. In the state of Andhra Pradesh it was found that "among the identified factors, male circumcision was estimated to have the highest relative impact on reducing HIV per unit of population" [Dandona et al., 2008]. This study involved 13,838 people aged 15-49 from 32 rural and 34 urban clusters. HIV prevalence was 1.7%. The proportion of uncircumcised men was 84%. Adjusted odds of having HIV after multiple logistic regression was 2.49 for uncircumcised versus circumcised men. As in the study above, odds of having HIV was higher for those who reported condom use for sex always or often. It was pointed out that few in India get circumcised for reasons other than religion, with 2.9% of non-Muslims and 96.4% of Muslims being circumcised. The clear benefits of circumcision have led to calls in India for physicians to inform patients in the interests of ethical responsibility [Madhivanan & Krupp, 2009].
Thailand:
In Thailand, a country with low circumcision rates, 1.4% of adults are infected, with 3 times more men than women infected. An early study of young military conscripts in Northern Thailand, where the men were having regular contact with female sex workers, found the rate of HIV was 1 in 18 to 1 in 32 [Mastro et al., 1994]. Thailand also has high infectivity in homosexual and injecting drug user groups [Normile, 2009].
Japan:
In 2007 there were 9,600 adults and children with HIV [http://www.avert.org/aids-asia.htm]. In 2006 there were 914 new infections, with 60% being in men who have sex with men.
Middle East:
In Muslim nations such as Egypt, Iran, Pakistan, Bangladesh and Indonesia there is a very low prevalence of HIV infections [Short, 2006]. The 0.1% HIV prevalence in Pakistan contrasts with 0.3% in neighboring India where circumcision is low [Khan, 2009]. In Kuwait, a study published in 2007 of 1,068 male and 28 female patients presenting for a range of STIs, over 99% of which were acquired heterosexually, found no HIV or syphilis [Al-Mutairi et al., 2007].
North Africa:
In countries such as Algeria over 80% of men are circumcised and HIV is present in only 0.1% of the population [Addanki et al., 2008]. Mauritania, despite its neighboring countries having high HIV, has maintained a moderate rate of HIV probably because 60% of its males are circumcised [Addanki et al., 2008].
Comoros (Indian Ocean)
This population has a high circumcision rate and only 1 of 3,990 individuals tested was infected with HIV [Dada et al., 2007]. Only 1 sex worker was HIV positive. Syphilis was also rare, but STIs not associated with circumcision status were prevalent.
Latin America:
At the end of 2007 there were 1.7 million people living with HIV in Latin America [http://www.avert.org/aidslatinamerica.htm] [Soto-Ramirez, 2008]. In 2007 there were 120,000 new cases and 70,000 deaths. Three-quarters of HIV infections in Rio de Janeiro State have been attributed to unprotected heterosexual intercourse [Eyer-Silva et al., 2007]. Over 90% were uncircumcised. But, as elsewhere, homosexual intercourse is a major contributor, the HIV incidence in men who have sex with men being 3.5% in Brazil and Peru [Sanchez et al., 2009]. This is higher than in MSM in the USA, where most MSM are circumcised. HIV is increasing among the heterosexual population [Soto-Ramirez, 2008]. A study in Rio de Jamiero of men, 75% of whom were heterosexual, found the protective effect of circumcision was 3-fold [Périssé et al., 2009].
Sub-Saharan Africa:
Sub-Saharan Africa has 68% (22.5 million) of the world's AIDS cases. Here 5% of 15-49 year-olds are infected [Sharlip, 2008]. In 9 southern African countries more than 12% of adults are infected [Potts et al., 2008]. Strikingly, in 4 of these, adult HIV prevalence exceeds 20%, the highest being Swaziland (33%) and Botswana (25%) [Potts et al., 2008; Bollinger et al., 2009]. It is a major problem for South Africa where over 5.5 million people are infected. Surprisingly, regions that have suffered more from conflict, genocide and rape (such as Rwanda, Congo and Angola) have a lower AIDS problem than wealthier, more literate countries such as those mentioned above [Potts et al., 2008]. There is also a strong positive correlation between the prevalence of HIV and wealth [Potts et al., 2008]. Amongst Kenyan women, HIV is 4% in the lowest economic quartile, but 12% in the highest [Potts et al., 2008].
HIV rates differ in different regions as a function of circumcision practise. Modelling has established that varying rates of circumcision have played a major role in explaining the strikingly different HIV epidemics in different parts of Sub-Saharan Africa [Orroth et al., 2007]. Circumcision rate of males over 15 years of age is 8% in Swaziland, 10% in Zimbabwe, 11% in Botswana, 12% in Malawi, 13% in Zambia, 14% in Uganda, 21% in Namibia, 25% in Uganda, 35% in South Africa, and 70% in Tanzania, 83% in Kenya, 85% in Ghana, 90% in Nigeria, 90% in Angola, 90% in the Democratic Republic of Congo, and 92% in Ethiopia (reviewed in: [World, 2008b; de Bruyn et al., 2010]).
Based on data from Kenya, if one partner is HIV positive and otherwise healthy then a single act of unprotected vaginal sex carries a 1 in 300 risk for a woman and as low as a 1 in 1000 risk for a man [Caldwell & Caldwell, 1996]. (The rates are very much higher for unprotected anal sex and intravenous injection.) This data did not take into account circumcision status. In Kenyan truck drivers female-to-male infectivity per sex act was 1 in 78 for uncircumcised and 1 in 200 for circumcised men [Baeten et al., 2005]. In Nairobi the rate is 1 in 1000 in the absence [Hayes et al., 1995] and 1 in 6 in the presence [Cameron et al., 1989] of genital ulcers.
UNAIDS data for 2004 show the prevalence of HIV in 38 Sub-Saharan African countries was 16% for the 8 countries with low (<20%) circumcision rates and 3% for the 22 with high (>80%) circumcision rates [Drain et al., 2006]. This was independent of Muslim and Christian religion.
Sub-Saharan Africa would appear to be where HIV first appeared in the human species. This region has 75% of HIV infections in the world [UNAIDS]. Of 44 sub-Saharan countries, in only 4 is the prevalence less than 1%. In 7 of the 16 in which it is greater than 10%, more than 20% of the population is infected. In South Africa 25% of adults are infected and in Botswana 40%. Mortality in those infected is elevated 50–500% [www.who.int/emc-hiv]. Sexual transmission continues to be by far the major mode of spread of HIV in Africa [Schmid et al., 2004]. Being in a stable sexual relationship with an HIV-infected person is a major risk factor for HIV infection [Malamba et al., 2005]. Naturally most of these infections involved uncircumcised men. The male, who is more likely to be promiscuous than the female, is the major source of infection in the majority of women, who only have that one partner [Fonck et al., 2000]. They may then pass on the virus to their children during pregnancy and breastfeeding. Men should therefore be the target for intervention strategies aimed at combating the disease.
There have now been over 45 studies of the role of circumcision in HIV incidence. One of the earliest key studies of the risk of HIV infection imposed by having a foreskin was that by Cameron, Plummer and associates published as a large article in Lancet in 1989 [Cameron et al., 1989]. It was conducted in Nairobi. Rather than look at the existing infection rate in each group, these workers followed HIV negative men until they became infected. The men were visiting prostitutes, numbering approx. 1,000, amongst whom there had been an explosive increase in the incidence of HIV from 4% in 1981 to 85% in 1986. These men were thus at high risk of exposure to HIV, as well as other STIs. From March to December 1987, 422 men were enrolled into the study. Of these, 51% had presented with genital ulcer disease (89% chancroid, 4% syphilis, 5% herpes) and the other 49% with urethritis (68% being gonorrhea). 12% were initially positive for HIV-1. Amongst the whole group, 27% were not circumcised. The men were followed up each 2 weeks for 3 months and then monthly until March 1988. During this time 8% of 293 men seroconverted (i.e., 24 men), the mean time being 8 weeks. These displayed greater prostitute contact per month (risk ratio = 3), more presented with genital ulcers (risk ratio = 8; P < 0.001) and more were uncircumcised (risk ratio = 10; P < 0.001). Logistic regression analysis indicated that the risk of seroconversion was independently associated with being uncircumcised (risk ratio = 8.2; P < 0.0001), genital ulcers (risk ratio = 4.7; P = 0.02) and regular prostitute contact (risk ratio = 3.2; P = 0.02). The cumulative frequency of seroconversion was 18%, but was only 2% for men with no risk factors, compared to 53% for men with both risk factors. Only one circumcised man with no ulcer seroconverted. Thus 98% of seroconversion was associated with either or both cofactors. In 65% there appeared to be additive synergy, the reason being that ulcers increase infectivity for HIV. This involves increased viral shedding in the female genital tract of women with ulcers, where HIV-1 has been isolated from surface ulcers in the genital tract of HIV-1 infected women.
In this African study the rate of transmission of HIV following a single exposure was 13% (i.e., very much higher than in the USA). It was suggested that concomitant STIs, particularly chancroid [Caldwell & Caldwell, 1996], may increase the risk, but there could be other explanations as well. In uncircumcised males the highly vascular frenulum is particularly susceptible to tearing or other damage during intercourse, and is also a frequent site of lesions produced by other STIs [Szabo & Short, 2000]. The risk of HIV infection is thus further reduced by circumcision, which therefore reduces the synergy that normally exists between HIV and other STIs [Szabo & Short, 2000]. Prevalence of HIV was lower in circumcised men in Uganda, but the rate of other STIs was similar between circumcised and uncircumcised men, pointing to the preputial mucosa as an important target tissue for HIV, but not other STIs [Gray et al., 2004].
An earlier study in Nairobi was the first to notice that among 340 men being treated for STIs there was a 3-fold higher rate of positivity for HIV if they had genital ulcers or were uncircumcised (11% of these men had HIV) [Simonsen et al., 1988]. Subsequently another report showed that amongst 409 African ethnic groups spread over 37 countries the geographical distribution of circumcision practices indicated a correlation of lack of circumcision and high incidence of AIDS [Bongaarts et al., 1989]. In 1990 Moses in the International Journal of Epidemiology reported that amongst 700 African societies involving 140 locations and 41 countries there was a considerably lower incidence of HIV in those localities where circumcision was practiced [Moses et al., 1990; Moses et al., 1994]. Truck drivers, who generally exhibit more frequent prostitute contact, have shown a higher rate of HIV if uncircumcised [Rakwar et al., 1999].
Interestingly, in a West African setting, men who were circumcised but had residual foreskin were more likely to be HIV-2 positive than those in whom circumcision was complete [Pepin et al., 1992].
Of 33 cross-sectional studies to the mid 1990s, 22 reported statistically significant association, e.g. [Simonsen et al., 1988; Jessamine et al., 1990; Diallo et al., 1992; Hunter, 1993; De Vincenzi & Mertens, 1994; Urassa et al., 1997], by uni-variate and multi-variate analysis, between the presence of the foreskin and HIV infection (4 of these 33 were from the USA). Five reported a trend (including 1 of the studies in the USA) [Moses et al., 1994; Moses, 1996]. Of the 6 that saw no difference 4 were from Rwanda and 2 from Tanzania. A study of adolescent boys in Tanzania found that 16-22% who reported being circumcised were not, and that 39% of Muslims were not [Weiss et al., 2008b]. In an editorial review in 1994 of 26 studies it was pointed out that more work was needed in order to reduce potential biases in some of the previous data [De Vincenzi & Mertens, 1994]. Studies since then that did control for such potential confounding factors, have confirmed that there was indeed a significantly lower HIV prevalence among circumcised men [Urassa et al., 1997; Lavreys et al., 1999]. Hazard rate ratio for being uncircumcised in one of these was 4.0 [Lavreys et al., 1999].
Many of the earlier studies have now been re-evaluated and those that were negative are now consistent with the majority of studies, i.e., ALL studies show lower HIV in circumcised populations. In this large systematic meta-analysis published in 2000 [Weiss et al., 2000], 27 studies were examined, with 21 showing reduced risk in circumcised men. In 15 that were adjusted for potential confounding factors the association with circumcision was 0.42 (i.e., rate in uncircumcised was 2.4 fold higher). The difference was highest in men at high risk, HIV in circumcised men being 73% lower than in uncircumcised men (i.e., HIV was 3.7 fold higher for the uncircumcised). The authors concluded that safe services for circumcision should be provided as an AIDS prevention strategy in parts of Africa where men are not traditionally circumcised. Moreover, in tribes that do perform ritual circumcision, a study suggested transfer of HIV in infected blood contaminating the instruments used [Brewer et al., 2007]. Thus traditional circumcision needs to be made safer. Some of these infections may, however, have been from false reporting of sexual activity / ‘virgin status’ [Adams et al., 2007; Westreich et al., 2007].
The case-control studies have continued since then. A Kenyan study found HIV incidence of 8% in men overall, but 29% in uncircumcised men, and 17% in women [Sateren et al., 2006]. Amongst rural plantation workers HIV was reported in 2007 to be 2.9 times higher in men who were uncircumcised [Foglia et al., 2007]. A South African study found lower HIV in circumcised Xhosa men [Jewkes et al., 2006]. It found 3.6% had had sex with another man, mostly as a result of having been coerced, and was a one-off event. A study in 2006 in which circumcision reduced HIV incidence 8-fold found an unexpected association with hygiene [Meier et al., 2006]. Subpreputial wetness, an indicator of inferior hygiene, is associated with slightly higher HIV infection (prevalence relative risk = 1.4) [O'Farrell et al., 2006].
In addition to the many case-control studies there have been a number of prospective studies, including ones in Kenya and Tanzania, reporting statistically significant association with lack of circumcision. The increased risk in the significant studies ranged from 1.5 to 9.6. Later adjustment of the data for other factors showed all studies were significant in demonstrating higher HIV in uncircumcised men [Weiss et al., 2000]. Women are at higher risk if their partner is uncircumcised. A study in Dar es Salaam, Tanzania, where most men are circumcised, noted that married women, with one sex partner, had a 4-fold higher relative risk of HIV if their husband was uncircumcised [Kapiga et al., 1998]. A 36-month prospective closed observational cohort study of 2,400 adult plantation workers in rural Kenya found self-reported male circumcision was associated with a 68% reduction in risk of HIV infection [Shaffer et al., 2009].
In most of these studies circumcision status was self-reported. However, physical examination in one study showed that 33% of men who said they were circumcised were in fact not circumcised [Nnko et al., 2001]. Amongst Muslims, 26% were not circumcised. In the meta-analysis by Weiss and co-workers [Weiss et al., 2000], only one study actually verified the circumcision status by physical examination [Urassa et al., 1997]. Agreement between self-reported and actual circumcision status was only about 81% in a study in a small geographic area of Kenya [Brown et al., 2001]. This study also found many had only a partial circumcision due to enormous variation in operative technique used. Similarly, a study in Tanzania of schoolboys (mean age 15) found substantial inconsistencies in both self-reported and clinically-assessed circumcision status [Weiss et al., 2008b]. The authors point out that this may explain in part the lack of association between circumcision and HIV prevalence in some Tanzanian studies.
Inaccuracy of clinical reports of circumcision status can be the case especially if the clinician is a woman, as reported in a US study of White, Black and Hispanic males that showed a disagreement of 16% [Diseker et al., 2001].
A study of racially mixed adolescent males (mean age 15) in Houston, Texas found that only 69% of those who were circumcised knew this, with 7% thinking they weren’t and 23% unsure [Risser et al., 2004]. Thus the residual HIV infection amongst so-called circumcised groups could quite likely be to a large extent from this residue of uncircumcised men, i.e., the estimated protective effect from being circumcised could really be far greater than the statistics above.
A study in Sydney, however, involving Anglo homosexual men, found a 98% agreement between self-report of circumcision status and genital examination by a trained nurse [Templeton et al., 2008].
The conclusive findings emerging from the large number of studies have, moreover, led various workers to propose that circumcision be used as an important intervention strategy in order to reduce AIDS [Marx, 1989; Fink, 1990; Kirby et al., 1991; Hunter, 1993; Moses et al., 1994; Caldwell & Caldwell, 1996; Moses et al., 1998; Halperin & Bailey, 1999; Franco, 2004]. Such advice has been taken up, with, by 1999, the appearance of newspaper advertisements from clinics in Tanzania, western Kenya, Rwanda, Uganda and other parts of Africa offering this service to protect against AIDS [Halperin & Bailey, 1999]. Young men are opting for circumcision and tribal elders are changing the edicts of their culture by now allowing circumcision in order to prevent AIDS [Halperin & Bailey, 1999; Nnko et al., 2001]. In traditionally noncircumcising cultures, circumcision rate had increased to 23% overall with a mean age of having it done of 17.4 years, and the rate was even higher (57%) in those who had at least 8 years of education [Nnko et al., 2001]. Health was cited as the reason. The President of TRADAP (Traditional Doctors AIDS Project) stated that "When tradition and the health of our people are in conflict, it is the tradition we must sacrifice" [Green, 1994]. This early wisdom was reflected in the common knowledge of African tribal people about the benefits. It also pointed out that poverty had led unscrupulous men to pose as ritual circumcisers with devastating results.
Willingness to get circumcised is high [de Bruyn et al., 2009; Eaton & Kalichman, 2009]. The work in Tanzania [Nnko et al., 2001], as well as in all other studies such as in Kenya [Bailey et al., 2002], Botswana [Kebaabetswe et al., 2003] and South Africa [Lagarde et al., 2003; Rain-Taljaard et al., 2003], show the majority of population groups are willing to accept circumcision to reduce HIV.
A review of 13 studies from 9 countries that examined acceptability found a median of 65% of men (range 29-87%) were willing to become circumcised and 69% of women (range 47-79%) favored circumcision for their partners [Westercamp & Bailey, 2007]. Furthermore, 79% (50-90%) of men and 81% (70-90%) of women were willing to get their sons circumcised. In Botswana, 92% of mothers of newborn boys wanted their sons circumcised if the procedure was available in a clinical setting, with 85% saying the father must participate in the decision [Plank et al., 2009]. A study in South Africa found that women had a strong influence on the decision, often scheduling the appointment for their boyfriend or husband to get circumcised [Rain-Taljaard et al., 2003]. Interest in circumcision in the first roll-out programs in Africa was in part driven by women's preference for circumcised partners [Baeten et al., 2009a]. A circumcised partner also means the women are at lower risk of not just HIV, but Trichomonas vaginalis, bacterial vaginosis, genital herpes, human papillomavirus and Chlamydia (see earlier sections).
After 30 days 99% of men in a Kenyan study reported being very satisfied with the procedure, as were their partners (92%), and 96% had resumed general activities within the first week [Krieger et al., 2005; Krieger et al., 2007]. None of the men and only 0.3% of partners were very dissatisfied with the outcome. By 3 days 83% of those with regular employment had resumed working, and by 1 week this was 93%, rising to 99% by one month [Krieger et al., 2007]. By one month 10%, and by 3 months 65%, reported having had sex [Krieger et al., 2007].
Similar findings have been obtained in Zimbabwe [Halperin et al., 2005]. In the South African RCT 98.5% of men questioned 3 months after their circumcision were "very satisfied" with the result [Auvert et al., 2005] and in the Kenyan RCT 99.5% were "very satisfied" [Bailey et al., 2007].
Thus circumcision can be readily and successfully adapted into a culture. However, this must be accompanied by education that makes it clear that circumcision reduces, but does not eliminate the risk. In the "Four Cities Study", risk-taking behavior was highest where HIV prevalence was lowest and vice versa [Buvé et al., 2001]. Moreover, although earlier studies also appeared to show that circumcision is most effective as a preventative measure against HIV infection if it is performed prior to puberty [Kelly et al., 1999], subsequent work suggested a benefit at any age [Agot et al., 2004]. In the Kenyan study cost of supplies, obtained locally, equated to US$20, and charge for the procedure was US$13 in a government hospital and US$77 in a private hospital [Krieger et al., 2005]. Rigorous counselling against sexual activity until the wound healed was stressed.
The possibility of an absolute protective effect of circumcision in an otherwise healthy penis was suggested by a large study published in the prestigious New England Journal of Medicine in 2000 [Quinn et al., 2000]. This involved 415 heterosexual couples in which only one partner (228 men and 187 women) was HIV-positive. It followed them prospectively for 30 months. The incidence of seroconversion was 17 per 100 person-years among the 137 uncircumcised male partners. However, among the 50 circumcised men with a HIV-infected female partner, not one seroconverted, i.e., none became infected, even though they were having regular unprotected sex with an infected woman. The effect was apparent in circumcised non-Muslim men as well as Muslims (who wash after intercourse), suggesting behaviors arising from religion were not involved [Gray et al., 2000a]. Moreover, the protection was seen only when circumcision had been performed prior to puberty [Gray et al., 2000a]. A commentary to this article highlighted the need to explore circumcision in reducing the spread of AIDS [Cohen, 2000].
A study reported in 2004, in which fastidious matching of uncircumcised and circumcised groups was carried out, has continued to show a higher rate of HIV infection in uncircumcised men [Agot et al., 2004]. The study involved 845 Luo men in a single ethnic community in rural Kenya in which circumcision was dictated by their particular African-instituted Christian religious denomination, and involved 9 churches of each persuasion. In an accompanying Commentary on this article it was mentioned that "careful (even obsessive) statistical analysis has zealously controlled for every possible confounder", meaning that "the quality of the science informing the debate has just moved up a notch" [Franco, 2004].
Frequency of sexual intercourse has also been excluded. In a study of 188 circumcised and 177 uncircumcised men in Mbale, Uganda, non-Muslim circumcised men engaged in more risk-taking behaviors, such as drinking alcohol in conjunction with sex, sex with women on the first day of meeting, sex in exchange for money or gifts, pain on urination, penile discharge, earlier sexual debut (16 vs 17), more extramarital sex partners in the previous year (1.1 vs 0.6), and more nonwet sex [Bailey et al., 1999]. (The latter, which is also practiced in Haiti, the Dominican Republic and to a certain extent in the USA, in an uncircumcised man can cause bleeding of the foreskin and frenulum, so increasing infection risk [Halperin, 1999].) Muslims had a lower risk profile regarding all of these factors, except for being less likely to have used a condom ever or during the previous sexual encounter (odds ratio 0.3). This highlights the fact that the foreskin itself confers an increased risk of HIV infection. Research has also shown that differences in biological factors such as circumcision and STIs are more important than behavior in risk of HIV infection, with more people who considered themselves to be at low risk being infected with HIV [Johnson & Way, 2006].
Overall, rough estimates published in 1999 suggested that circumcision had prevented more than 10 million HIV infections by the end of that decade in Africa and Asia [Fischbacher, 1999]. Worldwide this figure would have obviously be greater.
An extensive Cochrane review in 2005 [Siegfried et al., 2005] examined 37 observational studies, and noted that these varied in quality and potential confounding variables, so making a meta-analysis inappropriate. It stated that although most studies showed a protective effect of circumcision, results of randomized controlled trials (RCTs) were needed. An earlier evaluation of the evidence by others had also advocated randomized controlled trials to cement the strong suggestive evidence [Bailey et al., 2001]. But in 2009 a subsequent Cochrane review, conducted after a very extensive analysis of data from the RCTs to be discussed below, concluded that "inclusion of male circumcision into current HIV prevention guidelines is warranted" [Siegfried et al., 2009]. "No further trials are required", the review stated. It seemed the results of the 3 RCTs "have now convinced the reviewers" [Roehr, 2009].
Three large randomized controlled trials (RCTs) in Africa:
Three randomized controlled trials were begun in the early 2000s. The results for one of these were reported in 2005 [Auvert et al., 2005]. This involved 3,274 uncircumcised men aged 18-24 in the Orange Farm area, a semi-urban region near Johannesburg in South Africa. The men were randomized into a control or intervention (circumcision) group and the intention was for evaluation at clinic visits at 3, 12 and 21 months. So striking was the benefit of circumcision that at 18 months the Data and Safety Monitoring Board stopped the trial early so that the control group could be offered circumcision without delay. Protection was 60% (or 61%, after controlling for behavioural factors such as sexual activity, which was higher in the intervention group). Thus circumcision “prevented 6 out of 10 potential infections”. In fact their per-protocol analysis (which corrects for the dilutional effect of cross-overs, so treating men who were actually circumcised as circumcised and men who were uncircumcised as uncircumcised, and is thus more meaningful) showed a protective effect of 76%. It was concluded that “circumcision provides a degree of protection against acquiring HIV infection equivalent to what a vaccine of high efficacy would have achieved” and “may provide an important way of reducing the spread of HIV infection”.
Moreover, 99% of the men were “very satisfied” with their circumcision.
The findings were consistent with the data from meta-analysis of observational studies above, but showed a higher protective effect.
The authors suggested that “if women are aware of the protective effect of male circumcision, this awareness could, in turn, have an impact on prevalence of male circumcision by encouraging males to become circumcised”. Also, circumcision “could be incorporated rapidly into the national plans of countries where most males are not circumcised” (just as the example of South Korea where circumcision has risen from virtually zero 50 years ago to 85% today [Kim et al., 1999]).
The authors further stated that circumcision "is an inexpensive means of prevention, performed only once, and ? over a wide age range, from childhood to adulthood" and "the number of HIV infections that could be avoided ? is high". Nevertheless, circumcision must be promoted as part of a package that includes safe-sex (condoms) and fidelity. Compare this with messages regarding prevention of cardiovascular disease, type 2 diabetes, cancer, etc, namely, stay slim AND don't smoke AND control blood pressure AND eat healthy food AND don't drink alcohol to excess, etc (i.e, not any of these alone). The study's findings were reported widely, including in two Science commentaries [Cohen, 2005b; Cohen, 2005a].
The two other randomized controlled trials, in Kenya and Uganda, that were to be completed in 2007 and 2008, were also stopped early (in Dec 2006) by the monitoring committee because the preventative effect was so striking. The findings were published in the esteemed medical journal the Lancet in Feb 2007 [Bailey et al., 2007; Gray et al., 2007a]. Circumcisions in the Kenyan trial were performed between Feb 2002 and Mar 2004 [Krieger et al., 2005]. Each study involved 2,784 and 4,996 uncircumcised men aged 18-24 and 15-40, respectively [Bailey et al., 2007; Gray et al., 2007a]. In each study half as many of the circumcised men became infected as the uncircumcised. The more relevant "as-treated" protective effect of circumcision was 60%. Follow-up data on men in the Kenyan trial indicated that by 3.5 years, not only was the protective effect sustained, but it was increased to 65% [Bailey et al., 2008b]. This is consistent with the suggestion that "early stopping may have underestimated the effect [of circumcision]" [Weiss et al., 2008a] and counters the suggestion that stopping a trial early may over-estimate treatment effects [Bassler et al., 2008]. Modeling has shown, moreover, that the effectiveness of the intervention (such as circumcision or condoms) is likely to be maintained over many exposures in the case infections with low transmission risk like of HIV [Wilson, 2010].
An as-treated meta-analysis of the initial findings from the three RCTs found that the protective effect was 65% [Weiss et al., 2008a]. This was identical to the summary risk ratio for the 15 observational studies that adjusted for potential confounders [Weiss et al., 2008a]. A meta-analysis by others of the RCT data found a relative risk reduction of 56% [Mills et al., 2008]. Yet another group, whose meta-analysis of 13 studies, 85% of which were from sub-Saharan Africa, obtained an overall risk ratio of 0.42 (ie, a 58% protective effect) [Byakika-Tusiime, 2008]. The adjusted risk ratio for randomized controlled trials was 0.43 and for observational studies was 0.39 (cohort studies 0.29 and case-control 0.54). For general populations it was 0.47 and for high-risk populations was 0.31. If circumcision status was ascertained by self-report the adjusted risk ratio was 0.55, but if by direct genital examination in the clinic was 0.35 (i.e., a 65% protective effect). The authors pointed out that the current data on male circumcision satisfies 6 of the 9 criteria of causality as outlined by Sir A.B. Hill, namely strength of association, consistency, temporality, coherence, biological plausibility, and experiment [Hill, 1965].
Among men at higher risk, risk reduction was higher, 70% [Gray et al., 2007a], being similar to the 71% protection seen in observational studies of high-risk populations [Weiss et al., 2000].
Extensive data analyses dismissed a plethora of other, potentially confounding, factors as contributing to the much higher HIV incidence in the uncircumcised group in each of the studies.
Only 1.5% [Bailey et al., 2007], 3.6% [Gray et al., 2007a] and 7.6% [Gray et al., 2007a] experienced an adverse event related to their circumcision, and these resolved quickly. There was moreover no behavioral risk compensation after circumcision [Bailey et al., 2007; Gray et al., 2007a].
Exploding the myth that circumcision is extremely painful, the Kisumu study found that at 3 days follow-up, 48% reported NO pain, 52% very mild pain, NONE moderate or severe pain, and by 8 days, 89% no pain, 11% mild [Bailey et al., 2007]. Moreover, 99.5% of the men were "very satisfied" with their circumcision and 0.5% were "somewhat satisfied". None were "dissatisfied".
The other myth dispelled by the RCTs was that circumcision diminishes penile sensitivity. In fact 64% of the men in the Kisumu trial reported an increase in their penile sensitivity and 54% reported greater ease in reaching orgasm [Krieger et al., 2008]. This did not mean an increase in premature ejaculation. The fact that circumcision does not impair, and for many may enhance, a man’s sensation and sexual pleasure, should reassure men considering whether to get circumcised [Sharlip, 2008].
The protective effect was greater than that reported conservatively in the abstract. For a start, it seemed that 3 of the men in the circumcised group were likely to have been HIV positive before the operation (since they tested positive at month 1 but had not had sex during this period, in accordance with study directions), and there was one HIV-positive man who disobeyed instructions and did have sex during the first month. After excluding these subjects, 2-year HIV incidence was 1.6% for the circumcised group vs. 4.2% for the control group = a 68% protective effect [Bailey et al., 2007].
In the Introduction of this paper the authors point out the fact that currently available prevention measures (barriers, etc) "have often been unsuccessful in restricting the spread of HIV", as well as to the futility of relying on a HIV vaccine, stating "there is little promise that an effective vaccine will be available within the next 15 years [Bailey et al., 2007]. They also noted that "although the availability of antiviral therapy for individuals infected with HIV is increasing worldwide, many more new infections are occurring for every additional person on such treatment." Antiviral treatment is horrendously expensive, whereas prevention is much more desirable, and circumcision is cheaper.
The data show that there was low condom use by each group, even when condoms were provided free to all participants. The low rate is very similar to what we see in developed countries. Those engaged in risky sex are prevalent in the same age bracket as ones who drive recklessly, binge drink, etc, etc. ... i.e., those in the age bracket where the 3 'I's apply ... 'immune, infertile, immortal'! Circumcision is a one-off and does not have to be 'applied', is not inconvenient (except for a few weeks of no sex after having it done), and reduces a plethora of other problems.
The new data (much as previous data) showed that circumcision reduces HIV infection irrespective of number of partners (where there was NO risk compensation, i.e., the circumcised men did NOT immediately go out and have more risky sex thinking that circumcision was a panacea for preventing HIV infection). In the South African randomized controlled trial the men who had a circumcision DID exhibit increased sexual activity, but this did not increase their infection. In fact the per-protocol protection was 76%! This is enormous in public health terms.
An analysis of data from the 3 trials found that early resumption of sexual intercourse after circumcision did not appear to increase risk of HIV infection [Mehta et al., 2009a]. In the Rakai trial 5.4% of participants reported early sex, as did 3.9% in the Kisumu trial, and 22.5% in the Orange Farm trial. Nevertheless, the power of this study was limited and men should be advised to delay intercourse until after they have healed.
As well, these data showed circumcision conferred a cumulative efficacy of 48% in reduction of genital ulcer disease in the circumcised group [Gray et al., 2007a], as reported previously in observational studies.
Gray and co-workers state (p. 665, col 2, para 3): "neonatal circumcision or circumcision of younger boys will provide a simpler, safer, and cheaper option ..." [Gray et al., 2007a]. Here they refer to HIV benefits only, neglecting to mention the 11-fold reduction in UTIs in the first year of life, as well as phimosis that affects 10% of uncircumcised boys, and the other problem, diseases and infections throughout the life of the male and his sexual partners such as cervical cancer, chlamydia, etc. Such benefits were recognized in a statement issued by WHO and UNAIDS, who pointed out that neonatal circumcision is simpler, cheaper and safer than adult circumcision [World, 2008b].
Cost-benefit and lives saved:
For a circumcision efficacy of 50% when HIV incidence is 1.3 per 100 person-years in uncircumcised men (as in Rakai) 35 surgeries would be needed to prevent one HIV infection over 10 years, if all underwent circumcision [Gray et al., 2007a]. For South Africa (3.8 per 100 person-years HIV incidence) far fewer circumcisions would be needed to prevent one HIV infection. In South Africa STIs accounted for over 26% of all deaths and over 5 million DALYs in 2000, more than 98% of this burden was due to HIV/AIDS. A model that incorporates backward bifurcation showed, using partial data from South Africa, that male circumcision at a 60% efficacy level could prevent 220,000 cases of HIV and 8,200 deaths in South Africa within a year [Podder et al., 2007]. It also showed that male circumcision, by itself, could significantly reduce, but not eliminate, HIV burden in a community. Disease elimination was, however, feasible when combined with anti-retrovirals and, to a lesser extent, condoms. Another study estimated, based on a 50% protection against HIV infection by circumcision, an increase in the rate of circumcision to 100% from the current 10% in Ndola, Zambia would reduce the prevalence of HIV in adults from 27% down to 7% [Cohen, 2005a]. Thus the effect could be quite striking.
A joint analysis in 2006 by the World Health Organization in Geneva, UNAIDS and other experts around the world found that in Sub-Saharan Africa circumcision could avert 2 million new infections and 0.3 million deaths over the subsequent 10 years, and in the 10 years after that a further 4 million new infections and 3 million deaths, a quarter of these being in South Africa [Williams et al., 2006]. It equated circumcision with condom use or a vaccine.
In 2009 the UNAIDS/WHO/SACEMA Expert Group on Modelling the Impact and Cost of Male Circumcision for HIV Prevention found that in high HIV prevalence settings, one HIV infection would be averted for every 5 to 15 male circumcisions, and that the cost to avert one infection ranged from US$150 to US$900 using a 10-year time horizon [Hankins et al., 2009]. Circumcising men who had not started sexual activity would lead to the greatest population benefit in the long-term. They also stated that circumcising both adult males and neonates would maximize the short- and long-term impact of circumcision on HIV incidence.
Another reputable analysis, based on the South African trial data, found that 1,000 circumcisions would prevent 308 HIV infections over 10 years at a cost of $181 per HIV infection averted (net savings = $2.4 million) [Kahn et al., 2006].
In Botswana, it has been estimated that scaling up adult and neonatal circumcision to 80% coverage by 2012 would avert 70,000 new HIV infections through to 2025, and save US$47 million during this period [Bollinger et al., 2009]. Cost per HIV infection averted would be US$689. For a target year of 2015 the number of HIV infections averted by 2025 would be 60,000.
Estimates of the economic and human resources required for 14 Sub-Saharan countries where circumcision is <80% and HIV >5%, and assuming >85% of men get circumcised show an initial 5-year cost of $922M (private) / $397M (public) [Auvert et al., 2007]. 1,912 circumcisers would be needed, i.e., 0.23 per 10,000 adults. In years 6-10 the number needed would reduce to 504 and cost to $208M/$84M. They reported that 5-8 circumcisions would be needed to prevent one HIV infection, and concluded that, although expensive, the roll-out of circumcision would be cost-effective overall and sustainable, and would have important benefits to public health.
Mathematical modeling has predicted that with 80% circumcision uptake, a 45-67% reduction in prevalence would be achieved in both men and women within a decade in African countries with high HIV prevalence, and with a 50% uptake, HIV would be reduced 25-41% [Nagelkerke et al., 2007]. Further modeling has predicted that for a 60% efficacy, 19 surgeries would prevent one HIV infection in both sexes at a cost per infection averted of $1,269 [Gray et al., 2007b]. Circumcision therefore appeared in this analysis to provide a cost-effective prevention strategy. Indeed, the reduction predicted would be sufficient to "abort the epidemic" [Gray et al., 2007b]. Modeling by Auvert and colleagues found that in the first 5 years 2,282 circumcisers would be needed per 10,000 adults to achieve 85% circumcision in 14 Sub-Saharan African countries [Auvert et al., 2008]. For years 6-10 this fell to 513. The 5-year cost was estimated as US$919 million. The net savings were $2.3 billion over 20 years. A modeling study a Sydney group predicted that by 2020 complete male circumcision in an average country could reduce HIV prevalence from 12% to 6%, and incidence from 13.5 seroconversions per thousand to 7.3 per thousand [Londish & Murray, 2007; Londish & Murray, 2008]. These workers reported that for Zimbabwe a HIV rate of 25% for no circumcision would be reduced to 13% for 100% circumcision. Risk compensation would need to increase 40% for the protective effect to be negated. In areas where HIV prevalence is high and circumcision rate is low, circumcision of the majority of men could result over time in a 45-67% reduction in HIV prevalence in the population [Moses, 2009]. A simulated intervention in which circumcision was increased from 25% to 75% over 5 years found that the cost-effectiveness over 2-50 years will be cost-saving for any adult age group, but that targeting men older than recommended by the United Nations Joint Programme on HIV/AIDS would improve cost-effectiveness [White et al., 2008a]. It was further suggested that circumcision has the potential to abate the epidemic over the next 10-20 years [Gray et al., 2008].
It would, however, require 4,000 surgeons or surgical technicians, working at a rate of 10 circumcisions per day for 250 days per year to do 10 million circumcisions a year, well short of the number of surgeons or surgical technicians in Africa [Sharlip, 2008]. More resources and training are therefore needed urgently. The world's healthcare community has a moral obligation to assist in the scale-up of circumcision services [Sharlip, 2008].
The cost of one circumcision procedure in Africa is US$30-60 for an adult, and one-third of this for a neonate [Martin et al., 2007; Auvert et al., 2008; Wamai et al., 2008]. A study by the Rwanda Ministry of Health has determined that circumcising newborn boys to prevent HIV later in life is more cost-effective than circumcising adult males [[Binagwaho et al., 2010]. They estimated that each neonatal circumcision would cost US$15, whereas each adolescent or adult male circumcision would cost US$59. Neonatal circumcision would save more money than its costs. This included cost savings of treating infections that are ten times higher for circumcising later. The authors "suggest that Rwanda should be simultaneously scaling up circumcision across a brad range of age groups, with a high priority to the very young".
The research findings have led to the conclusion that "circumcision must now be deemed to be a proven intervention for reducing the risk of heterosexually acquired HIV infection in adult men" [Gray et al., 2007a]. Dr Kevin de C ock, Head of the WHO's HIV/AIDS department has called the studies an "extraordinary development" and circumcision a "potent intervention in HIV prevention" [http://www.iht.com/bin/print.php?id=4728041]. As stated earlier, on March 28, 2007, the World Health Organization endorsed circumcision for prevention of HIV infection [WHO, 2007]. "Global expansion of male circumcision programs [is a] vital tool for control of HIV infection" [Sahasrabuddhe & Vermund, 2007].
In the USA, where the overall circumcision rate is 79%, neonatal circumcision was calculated to reduce the lifetime risk of HIV amongst all males by 16% (8% in white males and 21% in black males) [[Sansom et al., 2010]. It was also cost-saving, even after discounting at a rate of 3% per year. The net cost of newborn circumcision per quality-adjusted life year (QALY) saved was $87,792 for white males and was even better for black and Hispanic males. How much greater then would be the cost-benefit for settings elsewhere in the world where newborn male circumcision is lower (for example only 10%, plus or minus, in Australia, the U.K., Europe, South America, India, China and many other Asian countries)? And how much greater would be the savings by inclusion of other conditions and diseases prevented by neonatal male circumcision, where the paper states: "Our analysis has two limitations that, if considered, would make neonatal circumcision [even] more cost-effective. First, our analysis did not include other health benefits associated with the procedure. Lack of male circumcision has been associated with increased incldence of sexually transmitted ulcer disease, infant urinary tract infections, penile cancer, and cervical cancer in the female partners of uncircumcised men.
In developed countries such as the USA, antiretroviral drugs (HAART) are being used to slow the progress from HIV infection to AIDS, so leading to a reduction in AIDS-related mortality and longer survival [Quinn, 2008]. In the US, in the first years of the HIV/AIDS epidemic median survival was approx. 12 months from time of diagnosis of AIDS, but now, in the era of HAART, median survival has increased from 60 months in 1995 to a survival in 2006 of 85-90% by 6 years [Quinn, 2008]. But the problem is that with the shift of HIV/AIDS from a fatal to a chronic disease a new range of health complications are being seen, many of which are life-threatening [Quinn, 2008]. Even at a high level of adherence, failure of HART to suppress virus replication completely has led to development of resistance to some of the drugs, especially protease inhibitors. Another problem about keeping people alive for longer is that they will have longer to transmit the virus to others.
Rapidity of spread:
The sorts of health problems faced by the 'third-world', coupled with a lack of circumcision may account for the rapid spread of HIV through Asia [Weniger & Brown, 1996]. The reason for the big difference in apparent rate of transmission of HIV in Africa and Asia, where heterosexual exposure has led to a rapid spread through these populations and is the main method of transmission, compared with the very slow rate of penetration into the heterosexual community in the USA and Australia, could be related at least in part to a difference in the type of HIV-1 itself [Kunanusont et al., 1995]. In 1995 an article in Nature Medicine discussed findings concerning marked differences in the properties of different HIV-1 subtypes in different geographical locations [Osborne, 1995]. A class of HIV-1 termed 'clade E' is prevalent in Asia and differs from the 'clade B' found in developed countries in being more highly capable of infecting Langerhans cells found in the foreskin, so accounting for its ready transmission across mucosal membranes. The Langerhans cells are part of the immune system and in turn carry the HIV to the T-cells, whose numbers are then severely depleted by the virus as a key feature of AIDS. The arrival of the Asian strain in Australia was reported in Nov 1995 and has the potential to utilize the uncircumcised male as a vehicle. More vigorous promotion of circumcision is needed to help curtail infections.
Risk Compensation:
A worry of AIDS experts is that knowing that circumcision is protective against HIV infection many will be less concerned about engaging in risky behavior [Cassell et al., 2006]. This is known as 'risk compensation' and was referred to above. Thus circumcision should be promoted as part of a package of protections. Encouragingly, at stated earlier, risk compensation was not a concern in the RCTs testing circumcision for HIV prevention [Bailey et al., 2007; Gray et al., 2007a; Mattson et al., 2008]. In a Kenyan study men were less likely to engage in risky behaviours during the first year after being circumcised and thereafter there was no difference compared with uncircumcised men [Agot et al., 2007]. A very comprehensive analysis of risk compensation in these men found no significant difference in sexual risk behavior between those who were circumcised and those who were not [Mattson et al., 2008]. In the South African RCT, however, the men who received a circumcision had more sexual encounters, with all 5 sexual behavior factors being significantly higher in this group [Auvert et al., 2005]. Despite this, their HIV acquisition was no greater (in fact less) than in the other two trials. Men in the circumcised arm of the Uganda RCT were less likely to use condoms in the first 6 months after circumcision, but thereafter all risk behaviors were similar in the two groups [Gray et al., 2007a].
Washing After Sex:
In a large randomized controlled trial in Uganda, follow-up interviews found that 83% of men washed after intercourse, but they did not have lower HIV infection (1.7 versus 1.2 infections per patient years) [Makumbi et al., 2007]. HIV was significantly higher (2.3) in men who cleaned within 3 minutes, compared with men who waited 10 minutes (0.39). Incidence was 2.2 for washing only, 1.0 for cloth and washing, and 0.6 for use of a dry cloth. The authors cautioned against promotion of washing as an alternative to circumcision.
Washing of hands after defecation in Indian truck drivers was associated with being less likely to report genital symptoms [Schneider et al., 2009]. Such behavior likely reflects general attitude to hygiene and behaviours that influence health. An information motivation program improved hand washing, but there was no change in sexual risk-taking.
Ethical and Other Challenges:
Several ethical analyses have concluded that it is unethical to deny safe male circumcision services in high HIV settings [Benatar & Benatar, 2003; Lie et al., 2006; Clark et al., 2007; UNAIDS, 2008]. Professional ethicists have, moreover, argued that "Neonatal male circumcision is medically necessary and ethically imperative" [Clark et al., 2007]. Other ethicists have concluded that "non-therapeutic circumcision of infant boys is a suitable matter for parental consideration" [Benatar & Benatar, 2003]. A review from South Africa suggests that parents from all ethnic groups should be advised to have their sons circumcised medically at birth and that "to the objection that this flies in the face of long-standing cultural values, one may counter quite simply that cultures do, and should, change: and there is no better reason to change than in the interests of survival" [Hargrove, 2008].
Various obstacles have, however, been recognized that could impede the full roll-out of circumcision [Rennie et al., 2007]. These include cost, supply of sufficient competent circumcisers [Pincock, 2007], circumcision training, adequacy of clinics and resources, best age, risk compensation, conflict between individuals within families and society who want it done or who don't want it done, prioritization of individuals in the face of limited resources, education to ensure circumcision is not seen as a panacea, and ethical issues in relation to culture, such as obstacles to changing the culture, as well as the need to alter certain practices in cultures that do circumcise so as to make the procedure safer [Rennie et al., 2007]. The misinformation and spurious arguments promulgated by anti-circumcision activists, if naively believed, has the potential to do damage to the roll-out of circumcision. All of the claims by these individuals were shown to be fallacious in an extensive 48-author commentary by reputable experts from around the world [Wamai et al., 2008]. The present author was one of these. Ensuring safe circumcisions will require all stakeholders to work together [Meissner & Buso, 2007].
The challenges seem minor, however, when compared to the perceived benefit. Many concerns have little validity. For example, as far as acceptability is concerned, the WHO produced another report in 2007 that showed that most cultures are neutral on circumcision [World, 2008b]. Those generally opposed include Indians of Hindu or Seikh faiths, since being uncircumcised distinguishes them from Muslims in this region. To increase the speed of the roll-out of circumcision paramedical as well as traditional and religious figures may be enlisted and trained [Griffith et al., 2007]. A voucher system for circumcision has been suggested [Griffith et al., 2007]. The Bill & Melinda Gates Foundation has provided substantial donations to circumcision for HIV/AIDS prevention, but more is needed, as are doctors from other parts of the world so that they can supplement the efforts already underway in Africa.
It needs to be recognized, however, that increased uptake of circumcision might result in stigmatization of uncircumcised men as being less ‘safe’ [Karim, 2007].
Condoms:
Sexual transmission of HIV and other STIs should be reduced by use of barrier protection such as condoms. A feared AIDS epidemic resulted in media campaigns starting in the 1980s aimed at increased condom use. In a 1996 survey of American college students only 60% had used condoms in the previous 6 months and less than 50% definitely intended to use them in the next month [Beckman et al., 1996]. Amongst a general US population sample, 62% of adults in 1996 reported using condoms at previous intercourse outside of an ongoing relationship [Anderson et al., 1999]. A report in 2006 found that in the USA 16% of men and 24% of women never used condoms during heterosexual sex with a non-primary partner [Sanchez et al., 2006]. In a US survey of women attending STI clinics in Baltimore, consistent condom use was stated as 25%, with 48% saying there had been no condom use in the previous 14 days [Jadack et al., 2006]. However, testing for male DNA in the vagina showed that this was present in all, albeit higher in the no condom-use group [Jadack et al., 2006]. A review in the Lancet in 2000 reported condom use was 55% [Donovan & Ross, 2000]. Amongst younger people, an Australian study found only 25% always used condoms, with 25% never having used them [Kang et al., 2006]. In Mexico condom use was 51% in young men and 23% when reported by young women [Caballero-Hoyos & Villasenor-Sierra, 2001]. Consistent condom use was, moreover, only 30% [Caballero-Hoyos & Villasenor-Sierra, 2001]. In Mexican public school students, average age of sexual debut was 14 years, and of the 13,293 subjects in this study, 46% had an intermediate and 37% a high HIV/AIDS knowledge [Tapia-Aguirre et al., 2004]. Males with high knowledge were more likely to use condoms (odds ratio 1.4), whereas females in this category were less likely to (odds ratio 0.7) [Tapia-Aguirre et al., 2004]. In a randomized controlled trial in Rakai, Uganda, involving the female partners of HIV-positive men, it was found that, despite undergoing counselling to use condoms, 61% of the sexually active participants did not use them at all, 20% used them inconsistently, and only 20% used them always [Wawer et al., 2009]. At each follow-up visit in this 2 year trial, the majority did not use condoms. A study in Mysore, India, found ever having used a condom to be associated with higher HIV prevalence in men (OR 2.75) [Munro et al., 2008]. Only 12% of men reported ever having used a condom. In women, condom use at last sexual intercourse was associated with 10.5-fold higher HIV infection [Munro et al., 2008]. In explaining these findings, it seemed that people with high-risk behavior first get infected with HIV and, after learning that they have become infected, then begin to use condoms.
Thus at least half of the sexually-active population of western countries are not using condoms. Indeed, the message of condom campaigns can easily be forgotten, especially in the young, in whom passion will over-ride compliance on occasions. Young people represent the most sexually promiscuous, at-risk group. They are at an age when risk-taking behaviour is prevalent (cf. smoking in young people vis-a-vis the anti-smoking campaign, dangerous driving, alcohol and drug taking, stunts, etc). In the case of HIV too, this will have tragic consequences. Many young people do not use condoms and openly scoff at the idea, despite the health warnings. Indeed it may be a sign of machismo to the young adult. It is well-known that the three "I"s are represented in their behavior of being "infertile", "immortal", and "immune". Thus education is only part of the answer and where an additional simple procedure is available to reduce the risk, then logic dictates that it should be used. The result will be many lives saved.
Even when used, the method of condom use is often incorrect. Condoms may break during intercourse. There can also be strong cultural and esthetic objections to their use. Also, application of a condom to a circumcised penis is easier than to a penis with a foreskin.
In the prospective study referred to earlier of circumcised and uncircumcised men whose female partner was infected, condoms were made available continuously [Quinn et al., 2000]. However, in discussing this study it was pointed out that 89% of the men never used condoms and condom use did not appear to influence the overall rate of transmission of HIV [Szabo & Short, 2000]. Only circumcision status did. A review of 10 studies from Africa found that overall there was no association between condom use and reduced HIV infection, with two studies actually showing a positive association between use of a condom and HIV infection [Slaymaker, 2004; Lopman et al., 2008]! The Zimbabwe study showed in fact that there was little difference in individual characteristics and behaviors between those who acquire HIV infection and those who do not [Lopman et al., 2008]. Circumcision removes the tissue that is the entry point for HIV. Unless a condom is used during all sex play, including that prior to actual penetration, then the risk remains of contact between the inner lining of the foreskin and HIV-laden secretions, sperm (in the case of homosexual sex), cells or tissues of an infected sex partner.
Thus condom use is far from a panacea for HIV prevention, since exposure of the vulnerable foreskin to infected biological fluids could take place during foreplay prior to application of the condom.
The approach of condom usage, although worthy of promotion, is regarded as unreliable because of the fact that it requires consistent lifetime compliance [Sharlip, 2008].
A meta-analysis of all studies found there was no difference in condom use or number of partners between circumcised and uncircumcised men [Kawabama et al., 2018].
Diaphragms, used commonly by women as a contraceptive, provide no protection against HIV infection [Padian et al., 2007].
Pre-Exposure Prophylaxis:
In 2005 the Centers for Disease Control reported that approx. 7% of men who have sex with men took an antiretroviral agent before engaging in risky sex [Costello, 2005]. The efficacy of this tactic is unclear. In women, antiviral treatment did not reduce HIV infection [Peterson et al., 2007]. And in animal models, complete protection required high daily doses of at least two different antiretroviral agents [García-Lerma et al., 2008]. It also accelerated transmitted drug resistance in monkeys with breakthrough infections [García-Lerma et al., 2008]. In a computer simulation of HIV infection in men who have sex with men in the USA, pre-exposure prophylaxis was found to reduce lifetime HIV infection from 44% to 25%, and increase mean life expectancy from 39.9 to 40.7 years [Paltiel et al., 2009]. The authors concluded that this strategy was unlikely to confer sufficient benefits to justify the current costs of anti-retroviral drugs [Paltiel et al., 2009].
Application of estrogen cream to the foreskin to thicken it has also been suggested [Pask et al., 2008]. Estrogen cream could also be used as a condom lubricant [Pask et al., 2008]. There are no data on whether or not this might help reduce uptake of HIV by the foreskin, but the authors did find a 4-fold increase in keratin thickness within 24 hours in preliminary testing involving two men, and this persisted for 5 days [Pask et al., 2008]. There is, moreover, a potential side-effect in that estrogen might cause feminization or reduce sex drive in any man who does this. Since HIV can be captured on the foreskin and persist for 6 days or more [Wu et al., 2003], during which time the virus could be passed on to a sexual partner, this approach could in fact enhance, not block, HIV transmission [Laurence, 2008]. In female monkeys with low estrogen, a month of estrogen cream applied twice a week to the vagina, protected them 9-fold against being infected with HIV [Smith et al., 2004].
Could vaccination against genital herpes reduce HIV prevalence?
The RCTs in Uganda and Kenya found half as much genital ulcer disease (GUD) in men in the circumcised arm of the trial [Gray et al., 2007a; Sobngwi-Tambekou et al., 2009a; Tobian et al., 2009a]. Genital herpes (HSV-2) was the most common GUD. HSV-2 infection, by causing ulcers on the penis, increases risk of HIV infection in men [Freeman et al., 2006]. Models based on the Kenyan RCT data suggested that no more than 10-20% of the HIV infections prevented by circumcision were due to efficacy against STI [Desai et al., 2006; Boily et al., 2008]. It has been suggested that circumcision, by lowering HSV-2, should contribute to a lowering of HIV infection [Bailey & Mehta, 2009]. Could vaccination against HSV-2 also reduce HIV prevalence? A model assuming 70% vaccine coverage predicted a 30-40% decrease in HIV in Africa after 20 years, with up to 100 vaccinations being required to prevent one HIV infection [Freeman et al., 2008]. As mentioned earlier, there may be a synergy between HIV and HSV-2 infections, with mathematical modelling showing that HSV-2 in either partner increased female-to-male HIV transmission 3.0-fold [Mahiane et al., 2009]. While good in theory, HSV-2 suppressive therapy has, however, failed to decrease HIV acquisition in men, as seen in two RCTs also referred to above [Tobian & Quinn, 2009]. A RCT in women similarly found that HSV-2 suppressive therapy had no effect on them becoming infected with HIV [Watson-Jones et al., 2008]. Further analysis of RCT data from Uganda has, moreover, shown that only 11.2% of HIV infections are mediated by a reduction in GUD after circumcision, and only 8.6% by a reduction in HSV-2 incidence [Gray et al., 2009b]. Ulcers prevented by circumcision were mostly not from herpes, but rather from foreskin tearing during intercourse [Gray et al., 2009b]. Since circumcision, by removal of the delicate foreskin, would prevent such tearing a further explanation for the prevention of HIV infection by circumcision is evident. It seems, moreover, that the persistence and enrichment of HIV receptor-positive inflammatory cells in biopsies from healed genital lesion after HSV-2 infection would help explain the inability of anti-HSV-2 therapy to reduce HIV acquisition [Zhu et al., 2009].
Synergy with high-risk HPV infection?
In the RCT in South Africa, being positive for high-risk human papillomavirus in urethral swabs was associated with a 3.8-fold higher inclidence of HIV [Auvert et al., 2009a]. There was no association with low-risk HPV. High-risk HPV may be a marker for increased sexual activity (which would increase risk of HIV) or there may be a causal explanation.
Circumcision protects the insertive partner in homosexual anal intercourse:
In New York City, the epicenter of the US HIV epidemic, a 2008 report noted that the prevalence of HIV amongst men who have sex with men is 8.4% [Manning et al., 2007], which is higher than Kenya (7.4%) or Uganda (7.0%) [McKinney et al., 2008].
The earliest studies of men who have sex with men did not consider different sexual practices. Nevertheless, one of the earliest involving 1,993 homosexual men in Seattle, found HIV was 2.2-times higher in the 15% who were uncircumcised [Kreiss & Hopkins, 1993]. Another, involving 3,257 homosexual men in 6 US cities studied from 1995-1997, identified various risk factors, lack of circumcision once again being found to double the risk of acquiring HIV [Buchbinder et al., 2005]. A failed HIV vaccine trial stopped in 2007 noted that "infected men were less likely to be circumcised" [Fox, 2007]. No association between circumcision status and either HIV or syphilis infection in homosexual men was seen in a San Francisco study, although the authors noted that a large proportion of gay men practice both insertive and receptive anal intercourse [Mor et al., 2007]. The latter would negate the possibility of seeing an association with circumcision status.
A study in 2001 in Sydney of homosexual men found no association with circumcision status, but the authors noted that it was too small and had too many confounding factors to be capable of yielding a valid conclusion [Grulich et al., 2001]. After increasing the size of this cohort, the lack of association with circumcision status remained [Templeton et al., 2009a]. But when they looked at the data for men who only performed insertive anal sex, an 89% lower rate of HIV infection was seen [Templeton et al., 2009a]. A US study, involving only Black and Latino MSM was also negative, although men who were insertive-only had 25% and 48% lower HIV in each respective racial group [Millett et al., 2007]. This group then performed a meta-analysis of 18 studies and found HIV was 29% lower in insertive-only MSM [Millett et al., 2008]. Higher study quality was associated with a reduced odds of HIV infection of circumcised men. Prior to the introduction of antiretroviral therapy the protective effect was 53% [Millett et al., 2008].
In Soweto, South Africa, MSM had high rates of HIV, it being 34% in gay and 6.4% in bisexual men [Lane et al., 2009a]. Circumcision had a 5-fold protective effect against HIV infection in this population (OR 0.20; 95% CI 0.1-0.1; P<0.0001). Most (>60%) of the men in this study practiced insertive anal sex only.
Amongst 1,056 initially HIV-negative men who have sex with men in Lima, Peru, those who became infected with HIV were more likely to have acquired syphilis (31%) or HSV-2 (8%) and were 4.9 times less likely to be circumcised (4.2% versus 20.6%) [Sanchez et al., 2009]. Of those who did not have syphilis or herpes, 20.5% of uncircumcised became infected, whereas none of 12 circumcised men did.
A review concluded that only MSM who are insertive-only may be at lower risk of HIV infection [Templeton et al., 2009c]. Modeling by these authors in a resource-rich setting (Sydney, Australia) showed that circumcision of men who have sex with men, especially those who were insertive-only, would be cost-effective for HIV prevention, with one infection prevented for every 118 circumcisions for men in the insertive-only category [Anderson et al., 2009].
‘Docking’:
Homosexual men who engage in a form of mutual masturbation [Short, 2004a] known as 'docking', a sexual practice that requires the foreskin, are placing themselves at risk, often not knowing of the danger this puts them in if their partner is infected. This practice is forbidden on a gay dating message board called 'Frot Club' (http://www.heroichomosex.org/fc/fchome/). For men who engage in docking it is in their sexual interests to oppose circumcision, as many do, so supporting their sexual practice, and possibly ensuring an ongoing supply of uncircumcised partners. It should, however, be noted that many supporters of circumcision can also be found amongst the gay community. Not surprisingly, homosexual men are more polarized than other men (and perhaps women too) when it comes to which kind of penis they think is best.
Male circumcision status and risk to women:
Intuitively it has been thought that if a man is HIV-positive, whether he is circumcised or not should make little difference to whether a women he has sex with will become infected [Turner et al., 2007]. An exception was, however, found in the case of women from high-risk settings (hazards ratio = 0.16). If the male had been circumcised prior to puberty, transmission of HIV to the female partner was reduced (relative risk 0.49) [Gray et al., 2000a]. But if the circumcision was after puberty, then there was no difference in risk of HIV transmission to the female partner [Gray et al., 2000a].
A 2-year unblinded randomized controlled trial in Rakai, Uganda of the uninfected female partners of men who were already infected with HIV at the time of their circumcision was stopped early because of futility [Wawer et al., 2009]. Although all of the differences were not significantly different, it was of interest that at the first (6-month) follow-up visit, HIV infection was 28% for female partners of men who resumed sex before wound healing, as compared to 9.5% in the partners of men who delayed sex until healing was complete, suggesting that early resumption of sex could have been an important factor in the interpretation of these findings [Wawer et al., 2009].
A meta-analysis of all relevant studies found that male circumcision led to a non-significant reduction in risk to women of 20% (95% CI 0.54-1.19) [Weiss et al., 2009]. But this did not include a later study of 7 sites in eastern Africa that noted 38% lower HIV in women who had infected male partners who were circumcised [Baeten et al., 2009b; Baeten et al., 2010]. The apparent contradiction between findings from the observational studies and those from the randomized controlled trials may be a result of the former being to do with men circumcised before puberty, whereas the RCT data were from men circumcised as adults [[Tobian et al. 2010].
Irrespective, women will see indirect benefits of male circumcision because a decrease in the pool of infected men will, in the long-term, reduce HIV transmission to women [Chersich & Rees, 2008]. UNAIDS has estimated that, in Africa, for every 5% increase in male circumcision, HIV would decrease by 2% in women [Kagumire, 2008].
For more on protection afforded by male circumcision against risk of HIV infection in women see systematic reviews [Grund et al., 2017; Morris et al., 2019b].
Recommendation to US President, and NIH position:
A 100-page document prepared in 2005 by the ‘Presidential Advisory Council for HIV/AIDS’ entitled “Achieving an HIV-free Generation: Recommendations for a New American HIV Strategy” argues the case for circumcision in HIV prevention. This official advice was adopted by a 16:2 vote (with 1 abstention) by the Council and presented to the President and Secretary Leavitt. The effort was praised by Carol Thompson from the White House. The National Institutes of Health have reacted similarly in realizing that they must develop policy that accords with the research findings. The US President’s Emergency Plan for AIDS Research (PEPFAR) is one of the agencies that has provided funds for expansion of circumcision services in Africa.
Circumcision could extinguish the HIV epidemic:
Analysis of the dynamics of the epidemic suggests that heterosexual transmission of HIV probably peaked during the 1990s [Shelton et al., 2006]. Given its effectiveness, circumcision, if implemented widely, has the potential to drive the prevalence of HIV virtually to extinction [Gray et al., 2007b]. In fact, a "parachute approach" to evidence-based medicine has been suggested, where, in the case of HIV, in the face of good evidence and enormous potential benefits, large scale circumcision could have been started in the early 1990s when the link was already clear, rather than wait until 2007 after the findings from the 3 RCTs [Potts et al., 2006]. In the intervening period millions of lives would have been saved.
Heterosexual transmission was the initial, and remains the major, mode of transmission worldwide, lack of circumcision is a major contributing factor to the AIDS epidemic. Even though other modes of transmission are prevalent in developed countries, heterosexual transmission remains and may be especially relevant for men who visit counties with high HIV. Moreover, in some studies [Kelly et al., 1999; Gray et al., 2000a], but not in a more recent one [Agot et al., 2004], the effectiveness of circumcision in AIDS risk reduction was greater when performed prior to puberty.
‘ABC’:
Thus, to the old "ABC" (abstinence, behavior and condoms) [Coates et al., 2008] one can now invoke an ABC that reads "antivirals, barriers and CIRCUMCISION".
Call for action:
Some quotes from an article by Harvard expert Daniel Halperin and associates [Klausner et al., 2008] are pertinent: “Male circumcision is the only modality for prevention of HIV transmission that has been proven to work by the highest standards of scientific evidence”. Particularly in regions of high HIV prevalence “the majority of uncircumcised men want the procedure performed, and generally an even higher proportion of women in those regions would prefer to have a circumcised partner”. Professional, governmental and advocacy communities have been criticized for the “glacially slow introduction of male circumcision”. “If this were an actual vaccine, packaged with a pharmaceutical company logo and shiny labelling, few people would be deliberating … There would instead be massive mobilization.” “Unfortunately, in these times of profit-driven healthcare, no single entity stands to earn large sums of money from male circumcision. The procedure is not patented, owned or trademarked.” “Male circumcision works: it is as least as good as the HIV vaccine we have been waiting for, praying for and hoping to see in our lifetimes.”
And this from the Institute of Catholic Bioethics: “Mandating neonatal male circumcision is an effective therapy that has minimal risks, is cost efficient and will save human lives. To deny individuals access to this effective therapy is to deny them the dignity and respect all persons deserve. Neonatal male circumcision is medically necessary and ethically imperative” [Clark et al., 2007].
A 48-author commentary that included leading experts on HIV and circumcision from around the world, including researchers from leading universities such as Harvard and the University of California, WHO, UNAIDS, the World Bank, and the present author, concluded that "the time has come for urgent and decisive leadership, not circular and unscientific arguments about an intervention whose efficacy has been proven beyond reasonable doubt. As with other previously 'controversial' topics such as the link between cigarette smoking and lung cancer, it is time to move beyond debating the merits of this evidelnce in professional journals and other legitimate communication outlets and to start implementing effective programs for safe, voluntary MC and reproductive health in high HIV-prevalence regions" [Wamai et al., 2008]. Messages about the proven benefit afforded by male circumcision in HIV prevention do need to be integrated with messages about safe sex and other behaviors [Rotheram-Borus et al., 2009], even though behavioral factors and condoms have not had much of an impact in general populations.
(Footnote: Brian Morris, the author of this review, chaired the Circumcision session at the 4th International AIDS Society Conference in July 2007.)



